Developer of India’s controversial COVID-19 vaccine warns some to avoid the shot
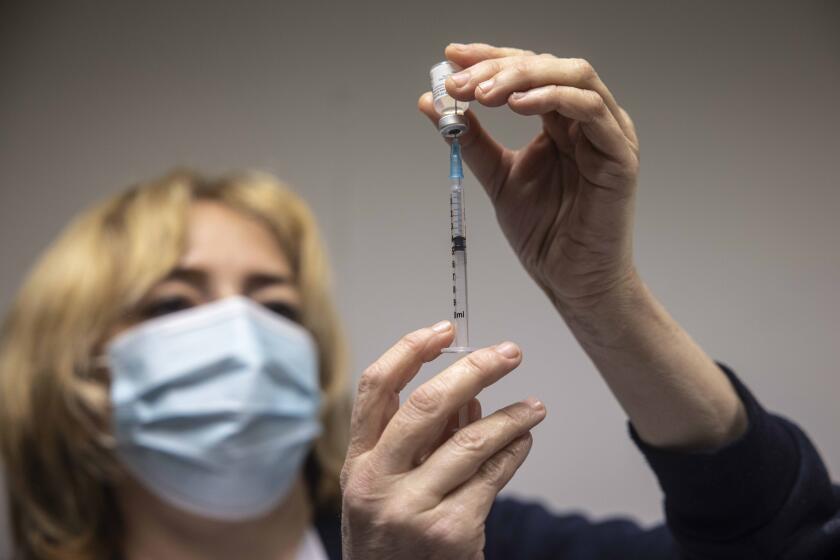
NEW DELHI — The company that developed India’s controversial homegrown COVID-19 vaccine is warning people with weak immunity and other medical conditions, such as allergies, fever and bleeding disorders, to consult a doctor before getting the inoculation — and, if possible, to avoid it altogether.
The company, Bharat Biotech, said Tuesday that those receiving COVID-19 vaccinations should disclose their medical condition, medicines they are taking and any history of allergies. It said severe allergic reactions among vaccine recipients may include difficulty breathing, swelling of the face and throat, rapid heartbeat, body rashes, dizziness and weakness.
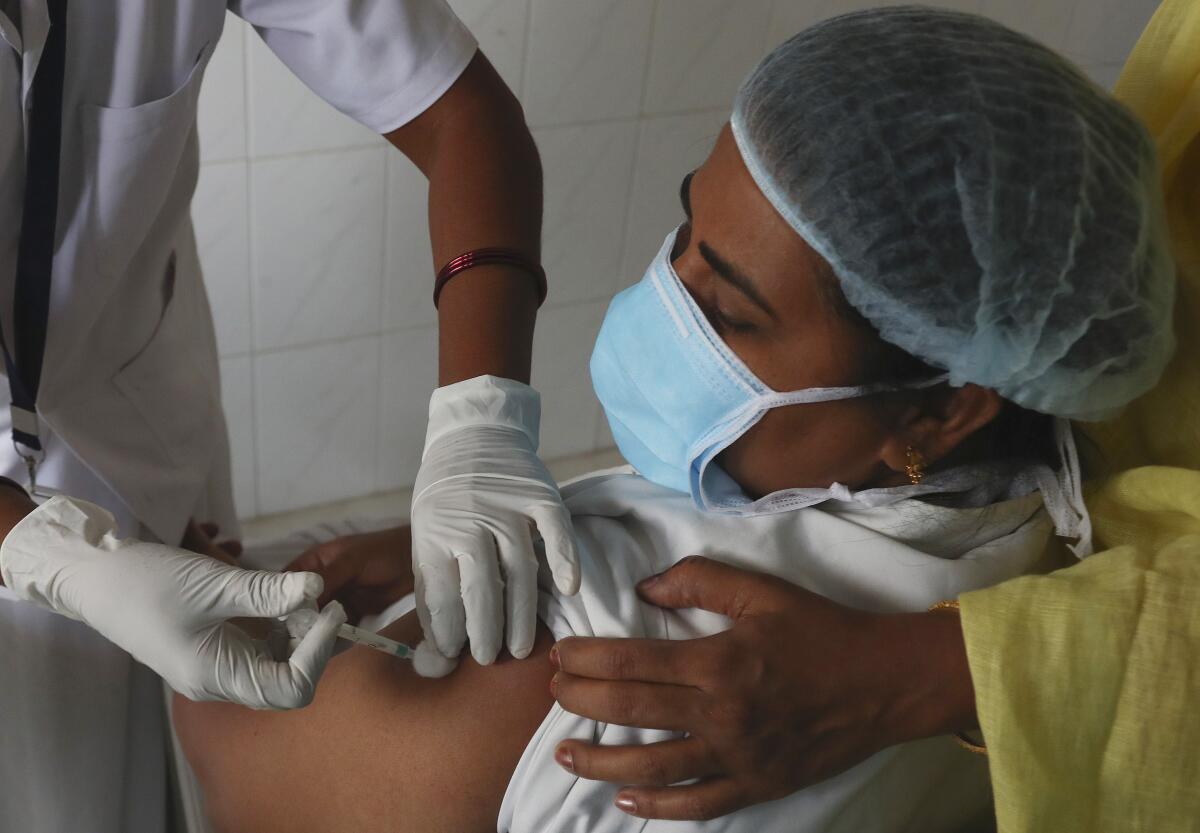
Bharat Biotech’s vaccine, Covaxin, ran into controversy after the Indian government allowed its use without disclosing concrete data showing its effectiveness in preventing COVID-19. Tens of thousands of people have been given the shot in the past three days after India started immunizing healthcare workers over the weekend in one of the world’s largest mass-vaccination campaigns.
India vaccinated 148,266 people Monday, taking its total to 381,305, the health ministry said.
Indian authorities hope to administer vaccines to 300 million people — a quarter of the country’s population. The recipients are to include 30 million doctors, nurses and other front-line workers, to be followed by 270 million people who either are over 50 or have illnesses that make them vulnerable to COVID-19.
Earlier this month, India approved the emergency use of two vaccines: one developed by British drugmaker AstraZeneca in partnership with Oxford University, the other by Bharat Biotech. The Indian drugs regulator took the step without publishing information about the latter’s efficacy.
Poor countries face long waits for injections as the U.S. and others buy up nearly all the global supply of Pfizer and Moderna vaccines.
Bharat Biotech has still not published data on its vaccine’s effectiveness but said it is complying with clinical trial guidelines.
The regulator maintains that Covaxin is safe and that it authorized the vaccine’s use in the belief that it could be more effective in tackling a new, more contagious variant of the coronavirus first detected in Britain. The regulator and the company have said efficacy data would be published after ongoing late clinical trials conclude.
Most hospitals in India are inoculating healthcare workers with the AstraZeneca vaccine, Covishield. But uptake in those hospitals using the Bharat Biotech vaccine has been relatively low, health officials said.
Hospitals in New Delhi that have been administering the Bharat Biotech vaccine have seen many doctors hesitate to get the shot.
Job losses, fear, and the burden of supporting families are exacting a heavy toll amid the coronavirus pandemic.
Dr. Vinod K. Paul, a member of NITI Aayog, a government think tank, described concerns about adverse effects of the vaccine as “unfounded and insignificant.”
“If our healthcare workers, especially doctors and nurses, are declining it, then it’s very unfortunate,” Paul told reporters. “Healthcare workers should have faith in our system.”
The vaccination drive began at a time when coronavirus infections in India have fallen sharply, and much of life has returned to normal.
India is second only to the U.S. in the number of confirmed coronavirus cases, with more than 10.5 million. The country ranks third in the number of reported deaths, behind the U.S. and Brazil, with over 152,000.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.