In mice, a single vaccine prompts the immune system to fight breast, lung and skin cancers
In the field of regenerative medicine, induced pluripotent stem cells have a lot of neat tricks up their sleeves. One of them may be teaching the immune system how to beat back cancer.
In research that could open a new frontier in the young field of cancer immunotherapy, Stanford University scientists have found that inoculating mice with stem cells that have been inactivated by radiation significantly boosted the animals’ defenses against cancers of the breast, lungs and skin.
And when administered alongside an immune booster already in use among humans, a vaccine of inactivated stem cells prevented cancer from recurring and spreading in mice that already had tumors surgically removed.
The vaccines that showed such promise were made with normal skin or blood cells taken from the mice and then reprogrammed to their primitive form, when they had the potential to become many different kinds of cells.
A vaccine based on these induced pluripotent stem cells, or iPSCs, is years from fruition at best, and may never pan out in the long run.
But if it does, it could be administered prophylactically to patients at high risk of developing cancers due to their genetic makeup or their risky behaviors like smoking. Or it might be administered to cancer patients after their tumor’s out-of-control growth has been disrupted by surgery, chemotherapy or radiation.
The study was published Thursday in the journal Cell Stem Cell.
The reprogrammed stems cells must be inactivated with a blast of radiation before they can be used in the vaccine. That crucial step reduces the risk that once inside the body, they will run amok and give rise to tumors.
Dr. Eirini Papapetrou, a cancer researcher at Icahn School of Medicine in New York, called the study’s results “very remarkable.”
A study in mice is a far cry from demonstrating that such a vaccine would protect humans from cancer, said Papapetrou, who was not involved in the new research. And as researchers aim to translate their findings to human treatments, they will have to show that inactivated stem cells are safe, she added.
But even if the Stanford team’s work does not produce a single stem-cell vaccine, it could identify better targets for immune-boosting cancer drugs to home in on, Papapetrou said. And that could make a new class of cancer drugs, called CAR-T immunotherapy, more effective.
In the first experiment described by the authors, lab mice received variants of the iPSC vaccine before researchers injected them with mouse breast cancer cells. After a week, all of the mice had developed tumors. But 70% of the animals vaccinated with the stem cells and the immune-booster cleared their tumors completely in four weeks. The remaining 30% had significantly smaller tumors than mice that were seeded with breast cancer cells, but got no vaccine.
In a second experiment, researchers inoculated mice with the iPSC vaccine and then injected them with mesothelioma cells, a form of lung cancer. Compared to mice that had not been vaccinated, those that got a mixture of stem cells and the immune booster mounted a robust defense against the cancer. In mice whose immune responses were strongest and most narrowly focused on the tumors, lung cancers were least likely to take hold and grow.
A third experiment using skin cancer cells demonstrated that an iPSC vaccine might be useful in preventing cancer recurrence or metastasis after an existing tumor has been surgically removed.
For this experiment, researchers infected mice with melanoma cells; after a tumor appeared, they performed surgery to remove it. At that point, some of the mice got the iPSC vaccine with an immune booster, and some got the immune booster only.
Mice that got neither treatment in the wake of surgery saw the rapid return of melanoma around the surgical site, as well as the appearance of metastases far from the original tumor. Mice that got the immune booster only maintained their tumor-free surgical sites, but suffered distant metastases. And the mice that got both the stem cells and the immune booster had no recurrence of tumors either close to or far from the surgical site.
How do stem cells teach the immune system to fight cancer?
Because pluripotent stem cells have the potential to give rise to most any kind of cell, they also carry inside them the seeds of cancer. Indeed, the very qualities of self-renewal that make them so promising as regenerative medicine treatments also give them the potential to give rise to tumors.
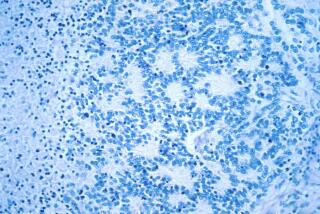
Stem cells in their most primitive form bear the outward signs of that kinship with cancer. Hundreds of distinctive little fragments of protein — called antigens — are expressed on their surfaces, and many of those fragments look just like some of the surface proteins expressed by cancer cells.
To Stanford physicians and cell biologists Joseph Wu and Nigel G. Kooreman, this outward likeness seemed like a teaching opportunity for the immune system, and a chance to add some firepower to the burgeoning field of cancer immunotherapy.
They surmised that if the immune system were trained to recognize the many proteins on stem cells, it would acquire a library of targets to draw on when it encountered cancer cells.
That would be a less-targeted approach than the one used by CAR-T immunotherapies. But it might prove more powerful, Wu and Kooreman figured.
In CAR-T treatments, doctors harvest some of a patient’s immune-system T-cells. Then they genetically engineer those defensive warriors to home in on a small handful of target proteins found on the surface of the patient’s own cancer. Finally, the population of these newly trained T-cells is multiplied and returned to the patient to fight that cancer.
But CAR-T’s effectiveness depends on scientists’ choice of just a few good targets for the immune system to go after. If the surface proteins they are trained to attack are not the right targets, or if cancer cells find a way to cloak those proteins from the immune system, they won’t work.
Perhaps showing the immune system all the proteins on the surface of a patient’s own stem cell would give it a whole bunch of targets to help it find and kill cancer cells. If one target protein wasn’t right, another one would be.
Over years of studying stem cells, Wu said he “could never answer which antigens” on a cancer cell’s surface would serve as the best beacons for immune cells. In the end, he said, “we probably don’t have to” identify which ones work best.
“There’s hundreds of tumor-associated antigens on the surface of these cells — thousands even,” Wu said. “There are probably ones we don’t even know about.”
MORE IN SCIENCE
The much-maligned flu shot has reduced the risk of serious illness this year by 36%, CDC says
About half the petroleum chemicals in L.A.’s smog come from products like soap and paint, not cars
In soil-dwelling bacteria, scientists find a new weapon to fight drug-resistant superbugs