Hailing a breakthrough in fighting cancer, FDA approves gene therapy that functions as a ‘living drug’
In a step that heralds a new era in cancer treatment, the U.S. Food and Drug Administration said Wednesday it has approved a form of gene therapy that is highly effective at fighting an aggressive form of leukemia in young patients with no other options.
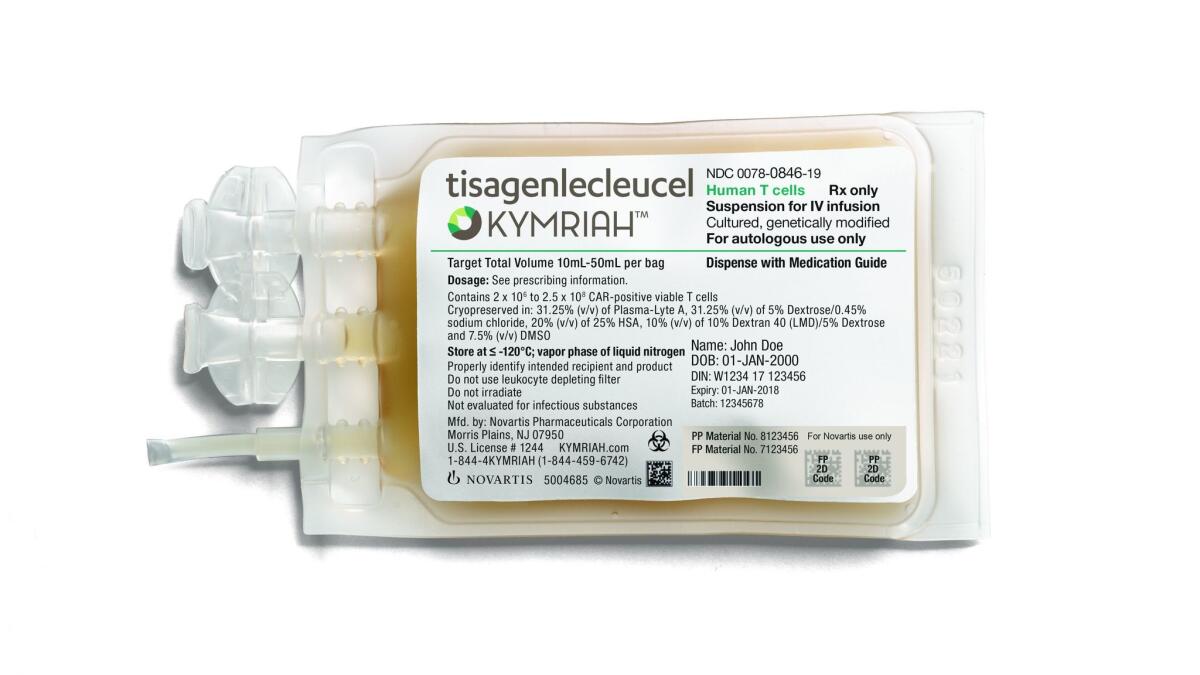
The treatment, to be marketed under the name Kymriah, is neither a pill nor an injection, but a personalized medicine service that functions as a “living drug.” Patients would have their body’s own disease-fighting T cells fortified and multiplied in a lab, then get the cells back to help them fight their cancer.
In clinical trials of 88 patients with a relapsing or treatment-resistant form of acute lymphoblastic leukemia, 73 went into remission after receiving the experimental treatment.
FDA Commissioner Scott Gottlieb, himself a survivor of blood cancer, predicted that this new approach to cancer treatment will “change the face of modern medicine.”
Cancer researchers and physicians outside the agency shared Gottlieb’s enthusiasm.
Dr. Crystal L. Mackall, associate director of Stanford University’s Cancer Institute, called Kymriah “a transformative therapy. … It represents an entirely new class of cancer therapies that holds promise for all cancer patients.”
Acute lymphoblastic leukemiais the most common form of pediatric cancer, affecting some 3,000 children and young adults yearly in the United States. Though it is considered highly curable in most patients, about 600 each year either do not respond to chemotherapy or see their leukemia return after an initial round of successful treatment.
“Those patients don’t make it — none of them do,” said Dr. Stephan A. Grupp, director of the cancer immunotherapy program at Children’s Hospital of Philadelphia, who administered the first course of Kymriah five years ago when it was an experimental treatment called CTL019.
That initial patient, 7-year-old Emily Whitehead of Philipsburg, Pa., saw her leukemia remit completely within three weeks of getting the treatment. Now 12, she was among those calling on the FDA to approve Kymriah for other patients like her.
“Certainly for blood cancers, this is a game-changer,” Grupp said. Adapting this therapy for patients with solid tumors, he said, will be “the work of the next five years.”
The new approach was designed to fight some of the most stubborn cancers by giving the body’s immune system a very specific assist.
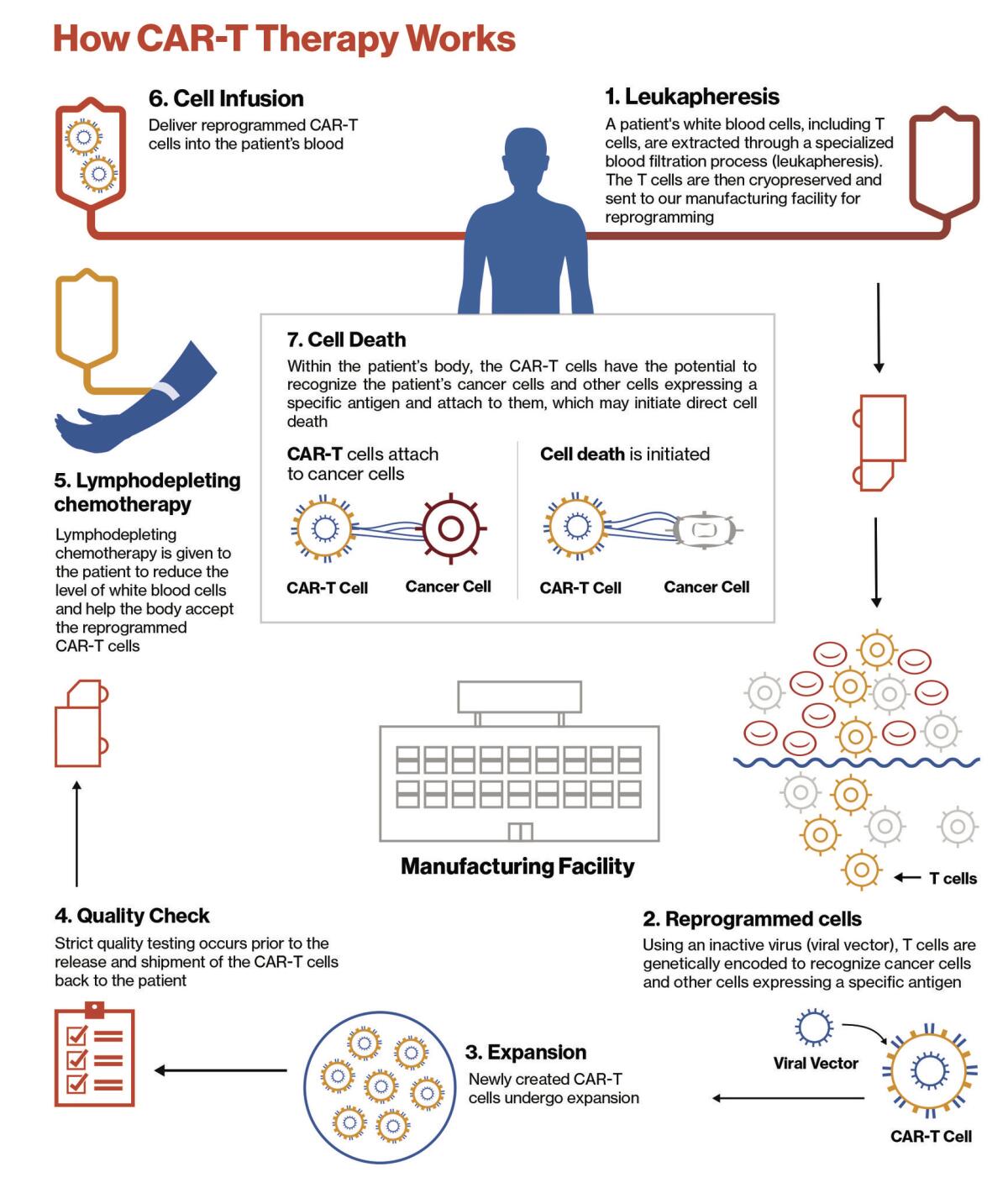
It starts by harvesting a cancer patient’s T cells, the warriors of the immune system. The cells are delivered to a specialized lab where scientists alter their DNA, essentially reprogramming them to target cancer cells. These reengineered cells are called chimeric antigen receptor T cells, or CAR-T cells.
The new and improved cells are copied millions of times before they’re sent back to the patient. Once infused into the bloodstream, the CAR-T cells are much better equipped to hunt down and kill cancer cells, wherever they may hide.
Novartis, the company that developed Kymriah, intends to have 32 certified treatment centers up and running by the end of 2018. Patients up to the age of 25 would go to one of these centers to have their T cells harvested and later reintroduced in their modified form.
The cells themselves will be genetically engineered at a Novartis manufacturing facility in Morris Plains, N.J.

Kymriah is the first CAR-T treatment to come before the FDA, but it won’t be the last. No fewer than 76 CAR-T treatments are currently under review at the FDA, and Gottlieb predicted that other approvals would follow.
Therapies that would operate in similar ways — engineering the immune system’s T cells to fight disease more effectively — are under investigation for a host of other conditions, including HIV/AIDS, genetic and autoimmune disorders and other forms of cancer.
“Today’s FDA ruling is a milestone,” said Dr. David Maloney, medical director of cellular immunotherapy at Fred Hutchinson Cancer Research Center in Seattle. “This is just the first of what will soon be many new immunotherapy-based treatments for a variety of cancers.”
Novartis, the Swiss pharmaceutical company that is gearing up to provide Kymriah to as many as 600 patients a year, said it would charge $475,000 for the treatment.
Novartis representatives said they calculated a “cost-effective price” for the therapy that fell between $600,000 and $750,000. But the company chose instead to charge a price that it said would “cover costs,” and to introduce a novel approach to billing. Chief Executive Joseph Jimenez said the company will not charge hospitals for the therapy if the patient does not fully respond in a given period of time.
The company also said it will launch a patient assistance program for those who are uninsured or underinsured, and provide some travel assistance for patients and caregivers seeking the treatment.
Gottlieb touted Kymriah’s approval as a turning point for the FDA as well. Novartis’ application for Kymriah came just seven months ago. The agency tagged the application with two designations that ensured its speedy review.
First proposed in 1972, the idea of correcting or enhancing genes to treat disease has a history buoyed by promise but also buffeted by failures. With recent advances in genomic medicine, cell biology and genetic engineering, efforts to locate and edit the genes and cells that play a key role in disease have injected new hope for such treatments.
Gene and cell therapies that target the immune system for enhancement have been particularly promising. They do, however, come with risks — specifically, that the activation of immune cells will run amok, sparking reactions ranging from rash and itching to fever and flu-like symptoms that can lead to death.
In approving Kymriah, the FDA warned that it has the potential to cause severe side effects, including cytokine release syndrome, an overreaction to the activation and proliferation of immune cells that causes high fever and flu-like symptoms, and neurological events. Both can be life-threatening. Kymriah can also cause serious infections, low blood pressure, acute kidney injury, fever and low oxygen levels.
The FDA called for continuing safety studies of the new therapy.
MORE IN SCIENCE
FDA cracks down on clinics selling unproven stem cell 'therapies'
Catastrophic storms, once rare, are almost routine. Is climate change to blame?
NASA’s Cassini spacecraft nears a fiery, brutal end, when it will plunge into Saturn




