Coronavirus Today: Rare clots pause Johnson & Johnson shots
Good evening. I’m Melody Petersen, and it’s Tuesday, April 13. Here’s what’s happening with the coronavirus in California and beyond.
California and other states have temporarily stopped administering Johnson & Johnson’s COVID-19 vaccine — a move urged by federal officials Tuesday following reports of six serious blood clots nationwide.
Gov. Gavin Newsom said the decision was made was “out of an abundance of caution” and noted that the drugmaker’s vaccine accounts for only about 4% of the total supply the state has recently received from the federal government.
California remains “on track to fully reopen” by its target of June 15, Newsom added.
Officials from the Food and Drug Administration and the Centers for Disease Control and Prevention said the pause may last only a few days, depending on what they learn in their review of medical data. They said it’s possible they will recommend that vaccinations with the company’s shots continue but adjust their guidance on who should get them.
The cases that prompted the warning involved six women ages 18 to 48 who developed a “rare and severe” blood clot called cerebral venous sinus thrombosis six to 13 days after receiving the vaccine, officials said. One of the women died, and another is in critical condition.
“Out of an abundance in caution, we’re recommending a pause in the use of the Johnson & Johnson COVID-19 vaccine,” said Dr. Janet Woodcock, the acting FDA commissioner. “We’re recommending this pause while we work together to fully understand these events and also so we can get information out to healthcare providers and vaccine recipients.”
She emphasized that reports of serious blood clots are “extremely rare,” but “COVID-19 vaccine safety is a top priority for the federal government, and we take all reports of adverse events after vaccination very seriously.”
Roughly 7 million Americans have received a dose of the company’s vaccine. That means the risk, if there is a link between the clots and the vaccine, is extremely low.
People who have received a dose of the J&J vaccine in recent weeks should contact their healthcare provider if they experience severe headaches, abdominal pain, leg pain or shortness of breath because those could be symptoms of the blood clot condition.
The CDC has scheduled an emergency meeting of its immunization advisory board for Wednesday to discuss the vaccine’s safety.
Johnson & Johnson said it was aware of the blood clot reports and was working with officials on the matter. “At present, no clear causal relationship has been established between these rare events and the Janssen COVID-19 vaccine,” the company said in a statement, referring to its division that developed the vaccine.
Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, said this type of blood clot is more difficult to address because the usual treatment — the anticoagulant drug heparin — can be dangerous in these cases.
The news came on the same day the city of L.A. opened eligibility for the vaccines to all residents 16 and older, a few days ahead of the state. Previously, all Californians age 50 and older were eligible, as well as those with qualifying medical conditions.
L.A. County health officials still plan to make that same move on Thursday and said the decision to pause Johnson & Johnson vaccinations would not slow them down. They said vaccine providers would be contacting patients who had been scheduled to receive the Johnson & Johnson vaccine to provide a new appointment for the Pfizer-BioNTech or Moderna shots.
One reason for the minimal impact to the rollout: California was already expecting shipments of the Johnson & Johnson shots to drop by 90% in coming weeks. The state was expecting an allocation this week of just 67,600 doses — down from 574,900 last week. The allocation was expected to fall even further next week, to 22,400.
By the numbers
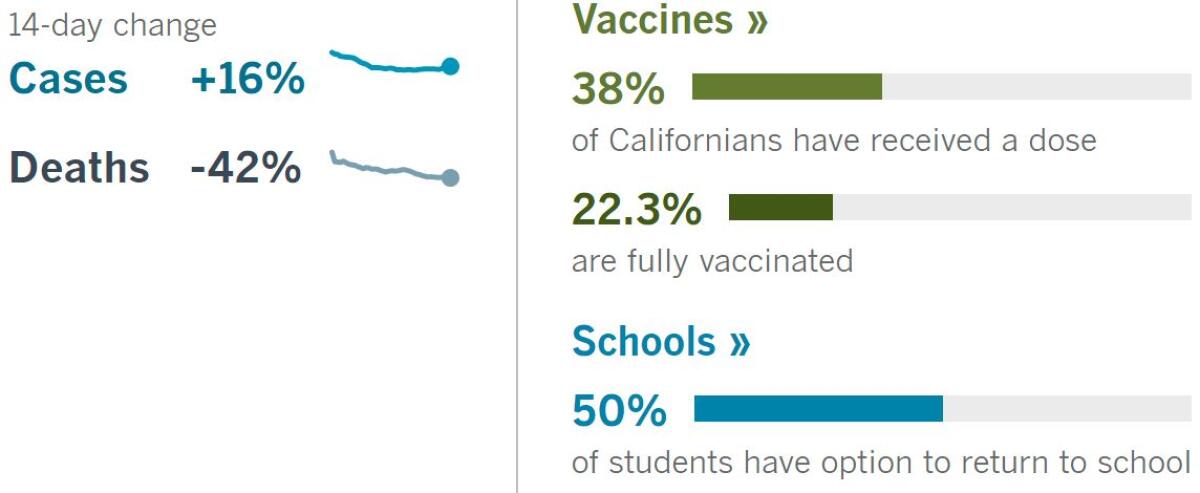
California cases, deaths and vaccinations as of 5:58 p.m. Tuesday:
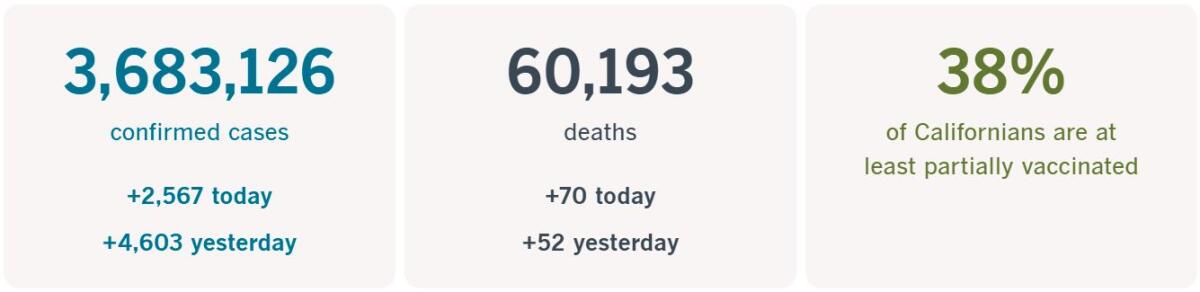
Track California’s coronavirus spread and vaccination efforts — including the latest numbers and how they break down — with our graphics.
Across California
After more than year of learning from laptops at home, some of the first students returned to their Los Angeles Unified School District classrooms. Sixty-one elementary and 11 early education schools reopened Tuesday, the first of some 1,400 campuses that will reopen by this month’s end in the nation’s second-largest school system.
At Heliotrope Avenue Elementary in Maywood, staff member Sylvia Vasquez asked each student how they felt as she did mandatory health checks. The answer she kept getting: “excited,” writes Times staff writer Howard Blume.
About 1 in 3 students were expected to return initially at Heliotrope. At some other campuses, the numbers are higher, but district survey data so far show fewer students are returning if they live in communities with high disease and death rates from COVID-19 — and Maywood was hit hard.
“I have some concerns,” said parent Fabiola Hermosillo. “But seeing how well organized they have it now, I felt confident to bring him back,” she said of her son Jesus, 6, who was going through his health screening.
L.A. schools Supt. Austin Beutner has hailed the reopening as a nation-leading model for school safety that is sensitive to families in low-income communities that have suffered the most from the pandemic. But the approach has also generated criticism from those who say the quantity and quality of instruction for the district’s 465,000 students have been sacrificed this year as a result of concerns by its teachers union.
Key safety provisions — including mandatory coronavirus testing as well as six-foot distancing between desks — go beyond what health authorities recommend. The six-foot rule sharply reduces the number of students allowed in a classroom at once, resulting in a schedule that allows elementary students to be in class on a half-time basis and has middle and high school students continuing lessons on laptops even when they return.
And the timing of reopening — about two months after elementary campuses became eligible to reopen under the state’s rules — was set to allow teachers and other district staff to achieve maximum vaccine immunity.
These decisions have defenders, including many parents who are still undecided about whether it is safe enough to send their children back to school.
Court documents reviewed by The Times — and connected to a September lawsuit over distance-learning — offer insights into how negotiators with the union, through an August agreement with the district, shaped instruction for the current school year.
The documents show, for example, that school district officials wanted a distance-learning program that more closely resembled a traditional school-day schedule. But the union pushed back: Too much screen time would be detrimental to students. Students and teachers would benefit more from a flexible schedule, which to the union meant a shorter school day and less required live online instruction.
The union said the resulting labor agreements comply with state law. Read Howard’s story for more.
Tuesday wasn’t just the start of in-person instruction for many LAUSD students. Also beginning is Ramadan, the holiest month of the year for Muslims in Southern California and around the world.
And with Ramadan comes fasting, abstaining from all food and drink during daylight hours. Typically, the fast also includes medications. But what about vaccines?
According to Omar Ricci, spokesperson for the Islamic Center of Southern California, a majority of Islamic scholars agree that getting vaccinated does not constitute breaking your fast and it is permissible to receive a vaccine in the daytime during Ramadan.
“Preservation of life is paramount in the faith, and therefore, getting the vaccine is acceptable,” Ricci said. “It’s not providing you with any form of nutrition, it’s not remedying a sort of an immediate malady or sickness or whatever, and it’s doing something to help preserve your life. So the majority of scholars that I’ve read have said ... you can go ahead and get the vaccine.”
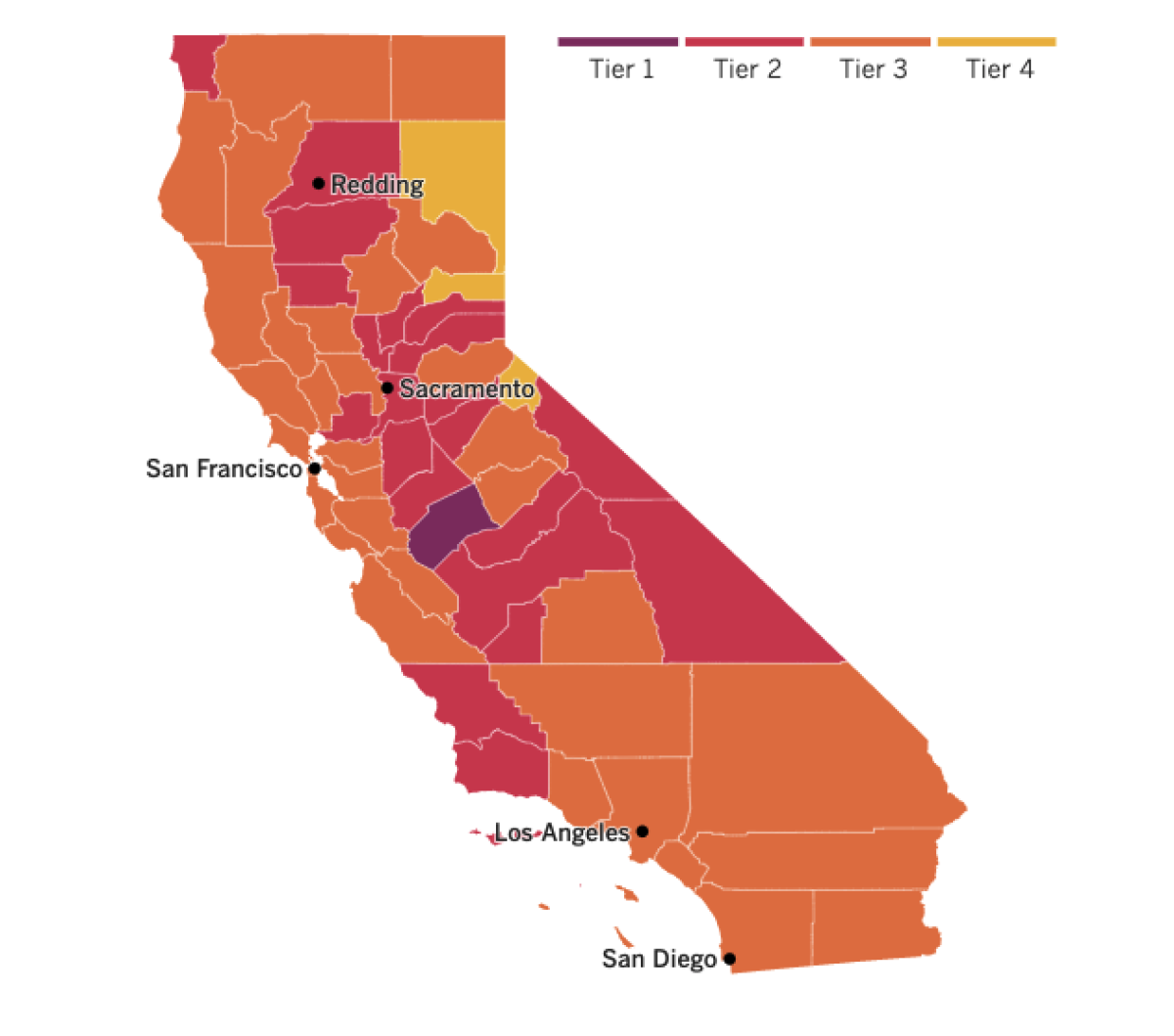
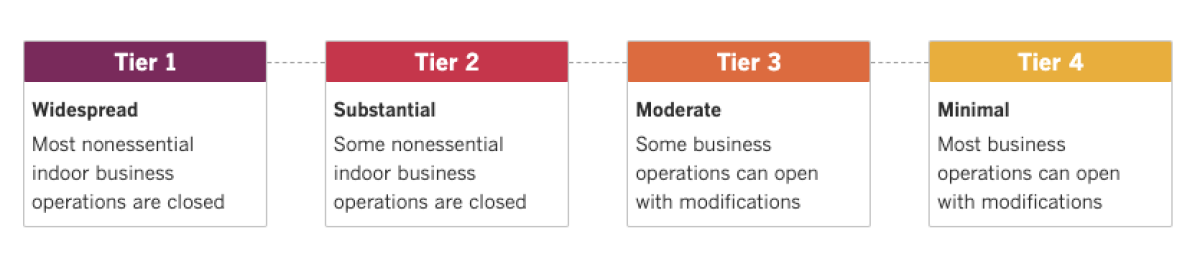
See the latest on California’s coronavirus closures and reopenings, and the metrics that inform them, with our tracker.
Consider subscribing to the Los Angeles Times
Your support helps us deliver the news that matters most. Become a subscriber.
Around the nation and the world
When the U.S. government last year enacted a temporary eviction ban, it did so through an unlikely agency: the CDC.
The public health agency has said the policy, recently extended through the end of June, helps stop the spread of the virus by limiting the number of tenants who lose their homes and are forced to move to shared housing, homeless shelters or the streets.
The moratorium has been praised by advocates for those at risk of being thrown out of their homes, but it has met stiff resistance from some landlords. Last month, a federal judge in Ohio concluded the agency lacked the authority to issue such a ban, the second such ruling.
And evictions are continuing in some places.
The CDC has said its authority comes from the Public Health Service Act, a nearly 80-year-old federal law that gives the federal government tools to stop the spread of communicable diseases.
The law is clear about some measures the CDC can take, such as ordering isolation and quarantine of people who have or may have the virus. But it’s less instructive on other measures, like the eviction moratorium, according to legal scholars.
Lawrence O. Gostin, a public health law expert at Georgetown University, said he believes the CDC has the legal authority to stop evictions, but says: “This is definitely a stretch because the Public Health Service Act doesn’t specifically mention evictions and traditionally CDC’s power doesn’t extend to housing.”
In another ongoing legal battle, women seeking abortion pills will no longer be required to visit a doctor or clinic during the pandemic to get them, U.S. health officials said Tuesday.
The FDA announced the policy change in a letter to the American College of Obstetricians and Gynecologists, one of several medical groups that has sued over the restriction put in place by the Trump administration.
Woodcock said an FDA review of recent studies found there was no increase in serious safety concerns when women took the pill without first visiting a doctor and discussing the drug’s potential risks, including internal bleeding.
The change clears the way for women to get a prescription for the pill — mifepristone — via telemedicine and receive it through the mail. However, abortion rights opponents are pushing legislation in several Republican-led states that would curb access.
Drug-induced abortion has been available in the U.S. since 2000, when the FDA approved the use of mifepristone. Taken with a hormone blocker called misoprostol, it constitutes the so-called abortion pill. About 40% of all abortions in the U.S. are now done through medication rather than surgery — an option that has become more pivotal during the pandemic.
In other news across the globe, India continues to battle a crippling surge of coronavirus infections that threatens to overwhelm hospitals in some cities.
The Indian government said Tuesday that it would approve the use of all COVID-19 vaccines that had been given an emergency nod by the World Health Organization or regulators in the United States, Europe, Britain or Japan.
India’s Health Ministry said the Russian Sputnik V vaccine had also been greenlighted for emergency use, and that safety will be monitored in the first 100 people who received these shots before their use can be expanded.
The decision was aimed at hastening the use of shots made in other countries and expanding the “basket of vaccines” available for domestic use, the ministry said.
The only way out of the crisis, experts said, is to vaccinate more people.
But the decision has global implications. India is a major vaccine producer, and its domestic needs have delayed the delivery of shots to the United Nations-backed initiative called Covax, which is trying to distribute vaccines equitably — including to poor nations that lack the power to negotiate with drugmakers.
India had earlier given the nod to the AstraZeneca vaccine made by Serum Institute of India and another one made by the Indian company Bharat Biotech.
Your questions answered
Today’s question comes from readers who want to know: I got the Johnson & Johnson vaccine. Should I be worried about a blood clot?
Generally speaking, the answer is no, my colleague Deborah Netburn reports.
If you are one of the roughly 7 million Americans who has received this particular COVID-19 vaccine, keep an eye out for possible symptoms of a dangerous blood clot, but remember that the condition is extremely rare and the odds are on your side.
Potential symptoms of the blood clot syndrome are abdominal pain, leg pain, severe headache and shortness of breath. If any of these occur within three weeks of getting your shot, contact your healthcare provider.
However, if you experience arm soreness or flu-like symptoms in the first few days after getting the vaccine, rest assured that those side effects are typical.
We want to hear from you. Email us your coronavirus questions, and we’ll do our best to answer them. Wondering if your question’s already been answered? Check out our archive here.
Resources
Need a vaccine? Keep in mind that supplies are limited, and getting one can be a challenge. Sign up for email updates, check your eligibility and, if you’re eligible, make an appointment where you live: City of Los Angeles | Los Angeles County | Kern County | Orange County | Riverside County | San Bernardino County | San Diego County | San Luis Obispo County | Santa Barbara County | Ventura County
Need more vaccine help? Talk to your healthcare provider. Call the state’s COVID-19 hotline at (833) 422-4255. And consult our county-by-county guides to getting vaccinated.
Practice social distancing using these tips, and wear a mask or two.
Watch for symptoms such as fever, cough, shortness of breath, chills, shaking with chills, muscle pain, headache, sore throat and loss of taste or smell. Here’s what to look for and when.
Need to get tested? Here’s where you can in L.A. County and around California.
Americans are hurting in many ways. We have advice for helping kids cope, resources for people experiencing domestic abuse and a newsletter to help you make ends meet.
We’ve answered hundreds of readers’ questions. Explore them in our archive here.
For our most up-to-date coverage, visit our homepage and our Health section, get our breaking news alerts, and follow us on Twitter and Instagram.




