Column: In blow to public health, judge tosses FDA lawsuit targeting a clinic offering unproven stem cell treatments
A federal judge in Riverside declared a California stem cell treatment firm to be exempt from Food and Drug Administration regulations, opening the door to the further proliferation of clinics offering therapies the FDA says are scientifically unproven and potentially dangerous.
In the ruling issued late Tuesday, Judge Jesus G. Bernal of Riverside declined to block California Stem Cell Treatment Center from continuing to offer purported stem cell treatments to customers.
Bernal accepted the center’s position that its treatments qualified for an exception from FDA regulations, in part because they were tantamount to surgical procedures.
Stem cells have been called everything from cure-alls to miracle treatments. But don’t believe the hype.
— Food and Drug Administration warns public against unproven stem cell treatments
Bernal’s ruling, which came more than a year after a seven-day trial in May 2021 and closing arguments last August, potentially undermines a years-long FDA crackdown on clinics claiming that stem cells can treat or cure conditions including orthopedic injuries, Alzheimer’s and Parkinson’s diseases, multiple sclerosis, and erectile dysfunction.
The “defendants are engaged in the practice of medicine,” Bernal ruled, “not the manufacture of pharmaceuticals.”
The FDA regulations, however, define drugs much more broadly than the “manufacture of pharmaceuticals” — rather, as any article that is “intended for use in the diagnosis, cure, mitigation, treatment, or prevention of disease.” Any such articles, the agency says, must have agency approval — and the California center’s treatments do not.
“To me, the language of his ruling sounded almost like it was written by the defendants,” Paul S. Knoepfler, a UC Davis stem cell biologist who has been tracking the proliferation of such clinics for years, observed in his laboratory blog.
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.
“The Bernal ruling will not be a good thing for careful oversight of stem cell clinic practices,” Knoepfler said. “It’s concerning to think about more people being put at risk.”
Knoepfler suggested that the FDA would feel duty-bound to appeal Bernal’s ruling in order to protect its regulatory campaign, and because it conflicts with federal court rulings in a nearly identical case the FDA brought against Florida clinics in Miami federal court.
The agency told me by email that it is “reviewing the court’s decision and does not have further comment at this time.”
Bioethicist Leigh Turner of UC Irvine, who has collaborated with Knoepfler on tracking the growth of the stem cell clinic industry, agreed that Bernal’s ruling is “an enormous setback for the FDA in an area where they’ve struggled for many years.... For people who work in this industry, it’s great news. But not for anyone concerned about patient safety, misinformation or disinformation.”
As part of its crackdown, the FDA has written hundreds of letters warning stem cell clinics that they’re violating the law, and has pursued some in court.
The FDA gave stem cell clinics three years to comply with its rules. The number of illegal clinics exploded.
After issuing regulations in 2017 declaring that treatments using unproven stem cell therapies were illegal, the FDA suspended its enforcement for more than three years to give clinic operators time to comply with FDA rules. The agency’s forbearance, however, opened the door to a further proliferation of suspect clinics.
By March 2021, according to a survey by Turner, nearly 1,500 U.S. businesses were pitching the suspect treatments at more than 2,700 clinics, reflecting a torrent of openings since 2016, when Turner and Knoepfler jointly started tracking the field.
“More than four times as many businesses than were identified five years ago are selling stem cell products that are not FDA-approved and lack convincing evidence of safety and efficacy,” Turner wrote last year.
Some clinics have charged customers more than $10,000 for the unproven treatments, plying the customers with unsupported claims of medical success. The fees are seldom, if ever, covered by health insurance. Some treatments resulted in serious medical complications.
The FDA also has warned the public that “some patients seeking cures and remedies are vulnerable to stem cell treatments that are illegal and potentially harmful.”
The FDA has approved stem cell treatments only for disorders of the blood-producing, or hematopoietic, system. No other stem cell treatment claims have been scientifically validated, the agency says.
“Stem cells have been called everything from cure-alls to miracle treatments,” the agency says in its public warning. “But don’t believe the hype.”
Bernal’s ruling conflicts with a 2019 decision by U.S. District Judge Ursula Ungaro of Miami, who ordered a Florida clinic shut down after the FDA asserted that its purported stem cell therapies were scientifically unproven and illegal.
Ungaro’s decision was upheld last year by the 11th Circuit U.S. Court of Appeals, which found that the clinic’s claim of exemption from FDA regulation on grounds similar to those claimed by the California center, didn’t apply. “No reasonable fact-finder could disagree,” the three-judge appellate panel ruled.
As it happens, in its finding the appellate panel specifically rejected an earlier ruling by Bernal, in which he denied the FDA’s request for a preliminary injunction against the California center and set the case for trial. The appellate ruling isn’t binding precedent for federal courts outside the 11th Circuit, which covers parts of the Southeast.
The FDA’s lawsuits against the Florida clinic and the California center were almost identical and filed on the same day, May 9, 2018. In both cases, the agency asserted that the clinics were in effect purveying illegal drugs as defined by federal law.
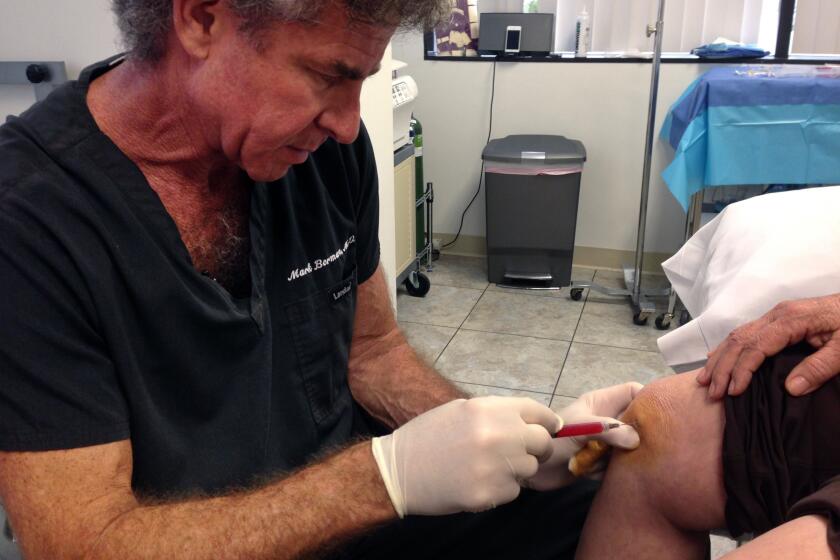
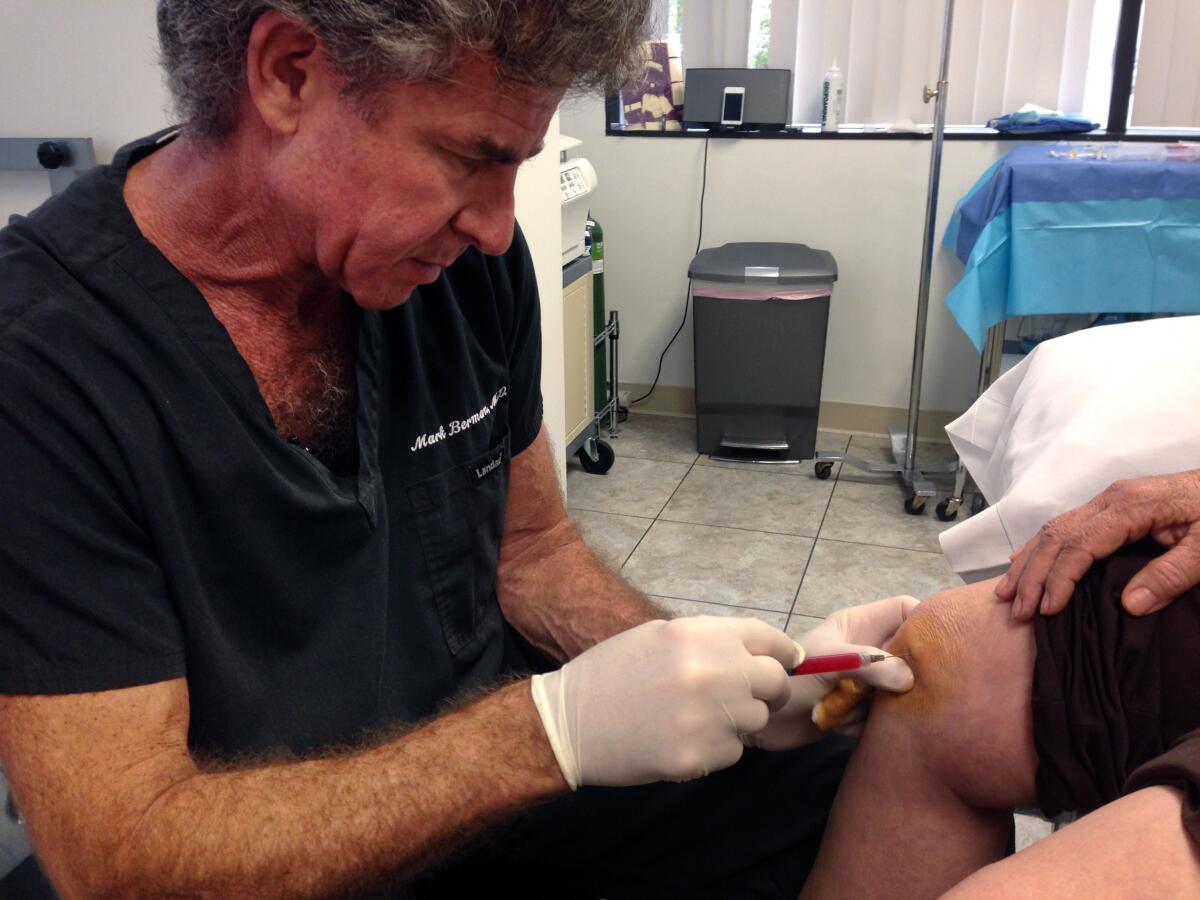
The lawsuits were part of an agency effort to clamp down on the burgeoning trade in stem cell-related therapies. The California lawsuit named the center’s founders, Elliott Lander and Mark Berman, as defendants. Berman died in April
Clinics with unproven stem cell treatments are already targeting COVID-19 fears.
The California Stem Cell Treatment Center has operated clinics in Beverly Hills and Rancho Mirage, and provides treatment protocols and other assistance to other clinics around the country.
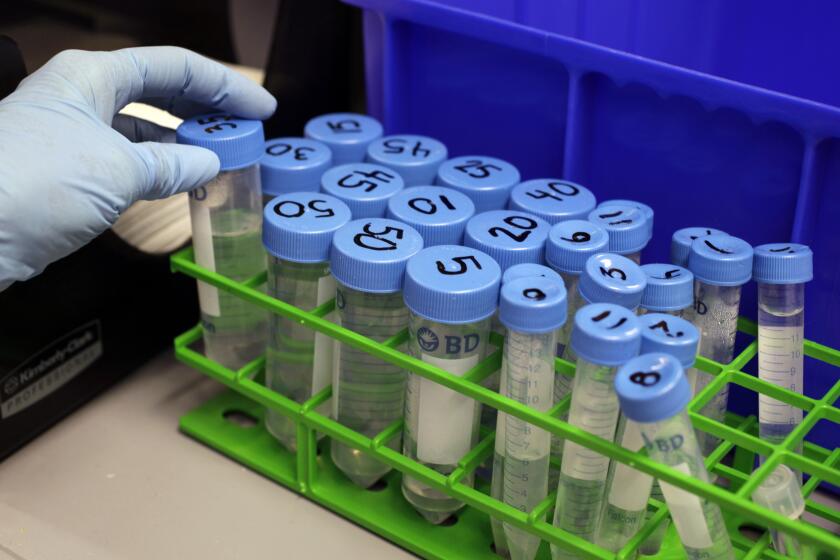
In its Florida and California lawsuits, the FDA targeted a widespread practice utilized by both clinics in which fat cells are extracted from a customer by liposuction. The extraction is treated to produce a fluid purportedly rich in stem cells known as a “stromal vascular fraction” or SVF, which is injected back into the same subject.
Mark Berman died last week awaiting a verdict in a federal lawsuit aiming to shut his stem cell practice down due to its use of unapproved and unlicensed treatments.
Both sets of defendants asserted in their defense that their activities qualified for exceptions from FDA drug regulations afforded to surgical procedures involving the use of a patient’s own tissues as well as the use of “minimally manipulated” tissues. The agency’s position is that the fat extractions are so heavily treated before the reinjections that the treatments fall outside those exceptions.
In his ruling, Bernal drew extensively and verbatim from California Stem Cell Treatment Center’s proposed findings of facts and scarcely at all from the FDA’s proposal. His ruling incorporated several scientific errors, according to Knoepfler.
For example, Bernal accepted the center’s assertion that “unlike manufactured drugs, the SVF Surgical Procedure does not create any cellular or tissue-based product that did not previously exist within the patient.”
In fact, Knoepler says, “there is no equivalent of SVF already in the body.”
More to Read
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.