Column: Lawsuits demanding ivermectin raise fears of judges ordering doctors to commit malpractice

Doctors and hospital administrators are facing yet another way in which the pandemic has shaken up social and medical norms: a string of lawsuits in which judges have ordered them to treat patients with ivermectin, a drug that lacks any scientific evidence of effectiveness.
In at least a half-dozen cases around the country, local judges have overruled hospitals or doctors who refused to administer the drug to patients with advanced COVID-19 disease. And more are in the works.
Ralph Lorigo, the Buffalo, N.Y., lawyer who has brought almost all these cases, says he has nearly 40 lawsuits in the works.
Public policy should not and does not support allowing a physician to try ‘any’ type of treatment on human beings.
— Ohio State Judge Michael Oster Jr.
“I get about 80 emails a day” seeking assistance, Lorigo says. “I’m the face of this litigation. I’m passionate about this because I truly believe in it.”
But legal experts and bioethicists say that judges who order treatments with unproven and unapproved drugs are overstepping the law and responsible judicial authority.
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.
“The question is whether the intervention being sought is within the standard of care, or rejected by the consensus of the medical profession,” says New York University bioethicist Arthur Caplan. “Ivermectin is as rejected as you could be. Nobody of any standing anywhere seems to believe it ought to be used as a COVID therapy. It’s pretty hard for a judge to tell a doctor to use it.”
But that’s happening.
Caplan is right about medical opinion on ivermectin as a COVID treatment. The drug was developed as an anti-parasite treatment, commonly used in a veterinary formulation to de-worm livestock and household pets, and to treat parasitic infections generally found in tropical regions. No valid scientific studies support its efficacy against COVID.
The Centers for Disease Control and Prevention, the Food and Drug Administration and the American Medical Assn. and other professional bodies all advise against it.
Even its leading U.S. manufacturer, Merck, says there is “no scientific basis for a potential therapeutic effect against COVID-19 from pre-clinical studies; no meaningful evidence for clinical activity or clinical efficacy in patients with COVID-19 disease; and a concerning lack of safety data in the majority of studies.”
But its cause has been taken up by anti-vaccine activists and conspiracy mongers who maintain that the drug industry and medical establishment have deliberately suppressed information about its efficacy.
A vocal ivermectin lobby has promoted the drug as a magic bullet against COVID, turning it into something of a political darling; radio host Joe Rogan recently claimed that ivermectin helped him defeat a case of COVID, though he listed several other nostrums as part of his therapy.
Ivermectin is increasingly the subject of misinformation and ignorance. It’s a troubling sign.
In his lawsuits and in a recent interview, Lorigo has repeated many of the ivermectin lobby’s talking points. He asserts that the pharmaceutical industry hasn’t conducted large-scale trials of ivermectin to establish its efficacy against COVID because as an old drug it can’t match the profits promised by COVID vaccines.
Lorigo suggests that hospitals that have fought him in court have pressured doctors, nurses or other affiliated professionals to refuse to prescribe the drug or dismiss it as a treatment.
“There’s no money in ivermectin,” he says. “They don’t want it to be the reasonable alternative to the vaccine.”
Lorigo contends that the National Institutes of Health has taken a “neutral” position on ivermectin, a point often made by ivermectin advocates. But that’s a simplistic interpretation of the NIH findings, which on balance are highly skeptical of the medication’s effect on COVID.
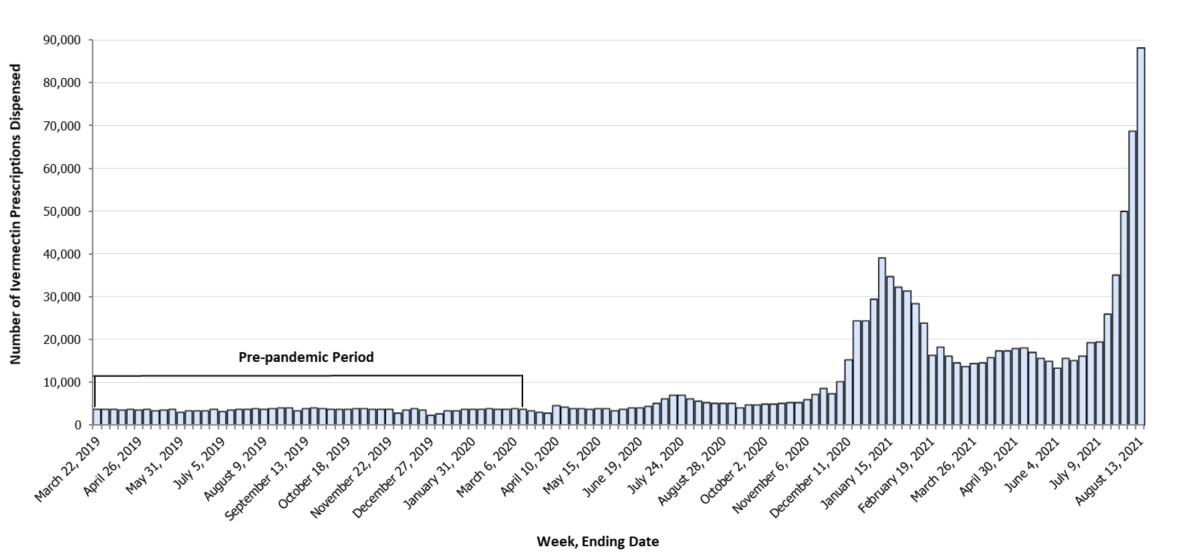
The NIH notes that doses of ivermectin necessary to replicate antiviral effects found in the lab would be as much as 100 times higher than doses approved for humans. Most studies reporting possible positive results, NIH says, have “significant methodological limitations.”
Among the flaws: They were too small; they were open-label trials, meaning that the researchers and participants knew what they were testing; patients received other drugs, making it impossible to distinguish any ivermectin effect; or they cited irrelevant clinical results. Others have found indications of fraud in some highly publicized studies promoting ivermectin.
The ivermectin lawsuits share some features with the “right-to-try” movement, which reached its climax in 2018 with the passage of several state laws and a federal law purporting to give desperately ill patients a crack at treatments that hadn’t passed full review by the Food and Drug Administration.
Anyone with a smidgen of knowledge about healthcare understood that the right-to-try legislation signed by President Trump on Wednesday was a scam, perpetrated by the Koch brothers and their henchmen.
Like those laws, the ivermectin lawsuits are based largely on an appeal to compassion; the idea is that once all accepted protocols have been tried and have failed, where’s the harm in one final roll of the dice, even on a treatment for which there’s no evidence of success?
As we reported at the time, however, right-to-try was never really about giving desperate patients a last chance; it was an effort financed by the Koch brothers to undermine the authority of the FDA as a regulatory agency. This was made clear by the federal law’s main sponsor, Sen. Ron Johnson (R-Wis.), after it was signed by President Trump.
“This law intends to diminish the FDA’s power over people’s lives, not increase it,” Johnson said.
In any event, desperate patients already had a path to obtaining unapproved medications — the FDA’s own expanded access program, which allows patients with immediately life-threatening illnesses to apply for access to drugs that have passed at least the agency’s very rough Phase 1 trials, which measure their safety.
In the last 10 years, according to the Congressional Research Service, the FDA has granted 99.3% of the more than 16,000 patient requests.
The right-to-try law essentially removed FDA approval from the process. But neither that law nor the FDA’s program can force drugmakers to provide the drugs, and most won’t unless they receive a green light from the FDA.
Indeed, for all the brouhaha, right-to-try laws appear to have been a bust. It isn’t known how many patients have sought and received drugs under right-to-try, but only two cases have been documented in the years since the federal law’s passage.
Ivermectin, touted as a treatment of COVID by the anti-vaccine crowd, has “no effect,” according to a major study.
Although it’s not unheard of for judges to issue orders in cases involving disputes over medical care, those generally involve disagreements over the application of accepted medical treatments. In a typical case, a judge will order a treatment to be continued or its withdrawal blocked until medical authorities and patients’ families resolve their differences.
One of the best-known cases involved Abraham Cherrix, a Virginia youth suffering from Hodgkin’s disease who at the age of 16 refused to continue chemotherapy, which had a 90% success rate, and opted for a folk remedy disapproved by the FDA. Concerned that Abraham was unduly influenced by his parents, who favored the alternative treatment, the state of Virginia charged the parents with medical neglect and sued to take custody of the teen and impose the accepted treatment.
The case was eventually resolved with an agreement that Abraham’s progress would be monitored by an oncologist. But once Abraham turned 18 and was able to make his own medical decisions, he opted for chemotherapy and as recently as 2017 was reported to be healthy and in remission.
Judges in the ivermectin lawsuits have been taking the opposite approach by ordering the administration of an unproven treatment — an “unprecedented step,” in the view of James M. Beck, a Philadelphia product liability lawyer who recently examined the case law on medical interventions for a legal blog. Federal judges have consistently held that patients have no “fundamental right to use any drug, regardless of its legality.”
That brings us back to Lorigo and his lawsuits.
In one sense, says University of Wisconsin bioethicist R. Alta Charo, judges’ orders at least insulate hospitals and doctors from the consequences of what might be considered malpractice — the prescribing of a drug without established evidence of safety or efficacy for the purpose.
“No reputable place is going to prescribe this stuff when they’ve been told directly by the FDA not to do it,” Charo says. “But at least they’re insulated from liability for medical malpractice because the court ordered them to commit medical malpractice.”
State medical boards are warning doctors not to spread COVID falsehoods, but will that stop them?
Lorigo hasn’t had unalloyed success in seeking court orders. In the most recent case, an Ohio state judge in suburban Cincinnati ordered a local hospital to administer ivermectin to a severely ill COVID patient; when none of the hospital’s own physicians agreed to do so, it was required to grant privileges to an Ohio doctor affiliated with an ivermectin lobbying group.
But that order was overturned by a second judge after two days of hearings on the scientific pros and cons of ivermectin. Judge Michael A. Oster Jr. concluded that “no single public health body in the United States supports the use of ivermectin to treat COVID-19” and that the outside doctor himself couldn’t say that the drug would benefit the patient. “Public policy should not and does not support allowing a physician to try ‘any’ type of treatment on human beings,” Oster wrote.
In some other cases, physicians who have treated the patients and have medical privileges at the hospital being sued have agreed to prescribe ivermectin, rendering the lawsuits moot. (Doctors are generally permitted to prescribe an FDA-approved drug for “off-label” use — that is, for a purpose for which it hasn’t been approved.)
In one case, a New York judge refused to order the treatment because there was no evidence that the doctor prescribing the drug had ever examined the patient or even reviewed his medical record. On the other hand, however, three of the hospital’s attending physicians “adamantly” opposed the treatment out of concerns that it might actually harm the patient.
What may be most dispiriting about judges trying to force doctors and hospitals into administering an unproven and probably useless medication for COVID-19 is how it plays into an alarming anti-science theme in American discourse.
“There’s this divide in the United States right now,” says bioethicist Leigh Turner of the University of Minnesota. “You have this mainstream consensus that ivermectin isn’t going to benefit individuals with COVID-19. But you have this very vocal, boisterous opposition that is convinced it’s helpful — conservative talk radio hosts, some physicians and this legal team that’s pushing these cases.
“They don’t really have evidence supporting what they’re doing, but they can go into court and have some success,” Turner says. “It’s not just yet another unproven drug being used for COVID-19, it’s court-mandated. It wouldn’t be shocking to see cases like this continue to crop up.”
More to Read
Get the latest from Michael Hiltzik
Commentary on economics and more from a Pulitzer Prize winner.
You may occasionally receive promotional content from the Los Angeles Times.















