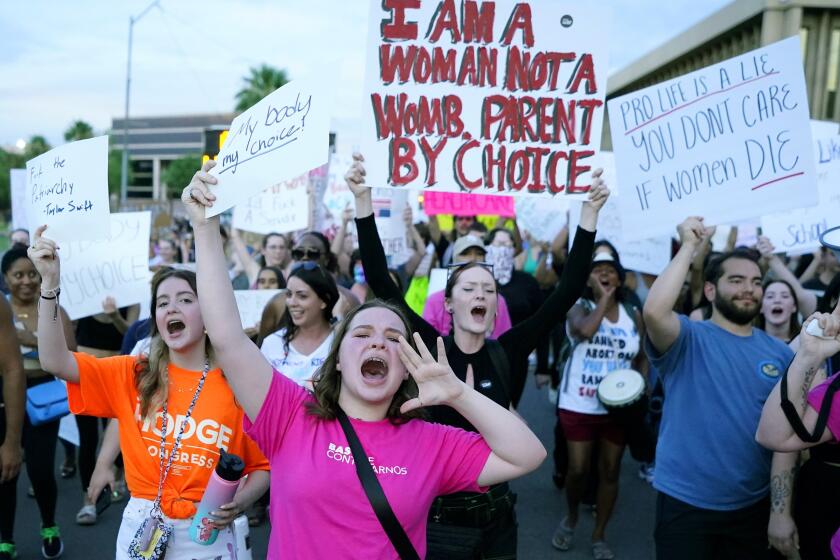Supreme Court upholds FDA’s approval of abortion pills for early pregnancies

WASHINGTON — The Supreme Court on Thursday dismissed a conservative Christian group’s claim that the abortion medication used by more than 5 million American women is unsafe and should be withdrawn from the market.
In a 9-0 decision, the justices said these antiabortion activists had no standing to sue. And they said conservative judges in Texas had no grounds for overturning the Food and Drug Administration’s regulations that allow for the mifepristone pills to be delivered by mail.
But because the court dismissed the case on standing, the decision does not prevent other antiabortion advocates from filing a similar lawsuit against the FDA, with different plaintiffs.
In case involving a California man, Supreme Court rejects trademarks that rely on a person’s name, including former President Trump.
“The plaintiffs have sincere legal, moral, ideological, and policy objections to elective abortion and to FDA’s relaxed regulation of mifepristone,” Justice Brett M. Kavanaugh wrote for the unanimous court. “But under Article III of the Constitution, those kinds of objections alone do not establish a justiciable case or controversy in federal court. Here, the plaintiffs have failed to demonstrate that FDA’s relaxed regulatory requirements likely would cause them to suffer an injury in fact. For that reason, the federal courts are the wrong forum for addressing the plaintiffs’ concerns about FDA’s actions.”
Last year, justices by a 7-2 vote blocked lower court rulings that would have made it harder for women to obtain the pills. Justices Neil M. Gorsuch, Amy Coney Barrett and Kavanaugh, the three appointees of Donald Trump who had voted to strike down the right to abortion, refused to join the antiabortion doctors who sought to restrict use of the abortion pills.
Justices Clarence Thomas and Samuel A. Alito Jr. dissented last year, but they joined Thursday’s ruling holding that the antiabortion doctors had no standing to sue.
The antiabortion doctors who sued do not prescribe abortion pills, and they do not perform abortions. They alleged, however, they should have standing because they might be required to perform an abortion if they were on duty in an emergency room when a patient arrived in distress after taking the abortion medication.
Kavanaugh said this concocted example did not reflect reality.
“Not only as a matter of law but also as a matter of fact, the federal conscience laws have protected pro-life doctors ever since FDA approved mifepristone in 2000,” he wrote. “The plaintiffs have not identified any instances where a doctor was required, notwithstanding conscience objections, to perform an abortion or to provide other abortion-related treatment that violated the doctor’s conscience.”
Abortion rights advocates said they were relieved by the outcome.
“I have both relief and anger about this decision,” said Nancy Northup, president of the Center for Reproductive Rights. “Thank goodness the Supreme Court rejected this unwarranted attempt to curtail access to medication abortion, but the fact remains that this meritless case should never have gotten this far.”
“We are relieved the Supreme Court didn’t take this bait, but unfortunately we know that this is far from the end of the line,” said Jennifer Dalven, director of the ACLU Reproductive Freedom Project. “Although the court refused to allow these particular people to bring this case, antiabortion politicians are waiting in the wings to attempt to continue pushing this case before an extremist judge in Texas in an effort to deny people access to medication abortion care.”
National medical groups also applauded the outcome.
The American Medical Assn. said the “efforts to second guess the FDA’s scientific judgment and roll back access to mifepristone were based on a sham case that ... relied on speculative allegations and ideological assertions to undermine decades of rigorous scientific review proving the drug is highly safe and effective for both termination of pregnancy and for medical management of miscarriage.”
The National Right to Life said the decision did not resolve concerns over the safety of the medication.
“Women remain unprotected from common complications with the abortion drug such as hemorrhage, infection, and failure to identify rupturing ectopic pregnancies in a timely manner,” said Carol Tobias, the group’s president. “While the abortion pill remains legal, we hope this battle to reinstate safety precautions will continue.”
It is not clear who might have standing to sue, but lawsuits against a drug or other medication usually begin with patients who complain they suffered severe and unexpected complications.
In 2000, the FDA approved the use of mifepristone as safe and effective for ending an early pregnancy. The pills are typically used in combination with a second drug, misoprostol.
In recent years, the agency relaxed its regulations for dispensing the pills. It did so based on studies that showed that serious complications were “exceedingly rare.” Under the new rules, pregnant women could obtain a prescription through telemedicine and receive the pills from a pharmacy or through the mail. And they could use the medication through 10 weeks of a pregnancy, up from seven weeks before.
The Guttmacher Institute reported that abortion medication was used in 63% of U.S. abortions last year.
Thursday’s decision does not change the law in states where abortions are illegal.
Shortly after the Supreme Court repealed the right to abortion in Dobbs vs. Jackson Women’s Health Organization, the Alliance Defending Freedom, a conservative Christian group, filed a lawsuit in Amarillo, Texas, seeking the repeal of the FDA’s approval of mifepristone. The plaintiffs were doctors and other healthcare activists from across the nation who oppose abortion and contend the drugs are unsafe.
Usually, plaintiffs have to show they were personally harmed by a federal law to have standing to sue. These doctors did not perform abortions or dispense the drugs, but they maintained that at least a few of the group’s members could be on duty in the future in an emergency room when a patient arrived suffering from complications after taking the abortion pills.
The group sued in Amarillo, knowing the case would come before U.S. District Judge Matthew Kacsmaryk, a Trump appointee and Christian legal activist before his appointment.
Mifepristone has been used more than 5 million times in the U.S. since its FDA approval in 2000. The Supreme Court will weigh a challenge to expanding its access.
As predicted, Kacsmaryk handed down a broad ruling last year. He ruled the antiabortion activists had standing to sue, and he ordered the FDA to suspend its approval of the drugs.
A few weeks later, the U.S. Court of Appeals for the 5th Circuit agreed to limit Kacsmaryk’s ruling. By a 2-1 vote, the appeals court said it was too late to unravel the approval of the drug in 2000, but not too late to overturn the FDA’s regulations that since 2016 have made it easier for women to obtain the pills.
Biden administration Solicitor Gen. Elizabeth B. Prelogar appealed to the Supreme Court and called the case a first.
She said it “marks the first time any court has restricted access to an FDA-approved drug by second-guessing FDA’s expert judgment about the conditions required to assure that drug’s safe use.”
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.