Tensions rise between AstraZeneca, EU over COVID-19 vaccine delivery

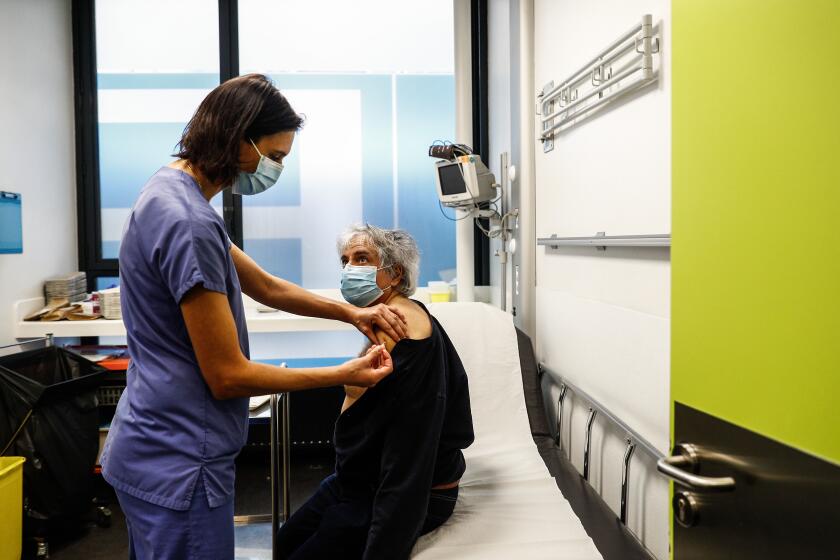
BRUSSELS — The European Union and drugmaker AstraZeneca sparred Wednesday over a delay in planned COVID-19 vaccine deliveries amid a deepening dispute that raises concerns about international competition for limited supplies of the shots needed to end the pandemic.
AstraZeneca Chief Executive Pascal Soriot addressed the dispute for the first time, rejecting the EU’s assertion that the company was failing to honor its commitments. Soriot said vaccine delivery figures in AstraZeneca’s contract with the 27-nation bloc were targets, not firm commitments, and the company was unable to meet them because of problems in rapidly expanding production capacity.
“Our contract is not a contractual commitment, it’s a best effort,” Soriot said in an interview with the Italian newspaper La Repubblica. “Basically, we said we’re going to try our best, but we can’t guarantee we’re going to succeed. In fact, getting there, we are a little bit delayed.”
AstraZeneca said last week that it planned to cut initial deliveries in the EU to 31 million doses from 80 million because of reduced yields from its manufacturing plants in Europe. The EU claimed Wednesday that it will receive even less than that — just one-quarter of the doses that member states were supposed to get during the January-to-March period.
The EU, which says it expects the company to deliver the full amount on time, on Monday threatened to put export controls on all vaccines made in its territory.
Stella Kyriakides, the European commissioner for health and food safety, rejected Soriot’s explanation for the delays, saying that “not being able to ensure manufacturing capacity is against the letter and spirit of our agreement.”
Kyriakides said AstraZeneca should provide vaccines from its U.K. facilities if its factories in the EU are unable to meet commitments. The comments are certain to create tension in the U.K., which completed its exit from the bloc less than a month ago.
“I call on AstraZeneca to engage fully to rebuild trust, to provide complete information and to live up to its contractual, societal and moral obligations,” she said at a news briefing in Brussels.
The EU’s contract with AstraZeneca is confidential and can’t be released without the agreement of both sides. The EU has asked AstraZeneca for permission to release the contract, Kyriakides said.
After a third round of talks aimed at resolving the dispute on Wednesday evening, she said she regretted the “continued lack of clarity on the delivery schedule” and urged AstraZeneca to come up with a clear plan for a quick delivery of the doses reserved by the EU for the first quarter. In a message posted on Twitter, Kyriakides however noted “a constructive tone” in the discussions with Soriot.
The dispute comes as the EU, which has 450 million citizens and the economic clout of the world’s biggest trading bloc, lags far behind countries such as Israel and Britain in delivering COVID-19 vaccines to its people.
The EU has signed deals for six different vaccines, but so far regulators have authorized only the use of two, one made by Pfizer-BioNTech and another by Moderna. The EU’s drug regulator will consider the AstraZeneca vaccine on Friday. The bloc is eager to roll out the AstraZeneca vaccine once it is approved.
Robert Yates, director of the global health program at the Chatham House think tank in London, said the EU-AstraZeneca dispute highlights the danger of “vaccine nationalism” as countries compete for limited supplies.
“For politicians, this is red hot. And, you know, unfortunately, what we’re seeing as well is that Brexit politics is playing into this,” he said.
“This is really, really bad news — not only bad news for the European countries involved,” he said. “I think what’s much worse is that these squabbles between rich countries potentially deny vaccines to people in the rest of the world.”
AstraZeneca is setting up more than a dozen regional supply chains worldwide to meet demand for its vaccine. Overall, it plans to deliver up to 3 billion doses to countries around the world by year’s end.
However, establishing each facility is a complicated process that involves training people and ensuring each batch of vaccine is safe and effective. Sometimes this goes smoothly, but in other cases there are problems, Soriot said.
“We train them on how to manufacture,” he said. “And then, you know, some people are new to this process.... They don’t know how to make the vaccine and they’re not as efficient as others.″
There are two basic steps in producing the vaccine. The first is a biological process that involves growing cells, which are injected with a virus, Soriot said. The second involves turning this “drug substance” into the final product, filling vials and testing each batch of vaccine.
Soriot said AstraZeneca had to reduce planned deliveries to the EU because plants in Europe had lower than expected yields from the biological process used to produce the vaccine. This has also happened in other regions as AstraZeneca sought to rapidly expand production capacity to meet demands from countries battling the pandemic.
The French are among the most reluctant people in the world to get a COVID-19 shot because of distrust of the government and past health scandals.
“We’ve also had teething issues like this in the U.K. supply chain,” Soriot said. “But the U.K. contract was signed three months before the European vaccine deal, so with the U.K. we have had an extra three months to fix all the glitches we experienced. As for Europe, we are three months behind in fixing those glitches.”
An official from the European Commission, the EU’s executive, said the bloc has agreed to give $407 million to AstraZeneca to develop its vaccine and deliver doses. The official, who wasn’t authorized to speak publicly, said the commission would be entitled to recover part of the money if the company fails to live up to the terms of this advance purchase agreement.
“We reject the logic of first come, first served,” Kyriakides said. “That may work at the neighborhood butchers, but not in contracts and not in our advance purchase agreements. There’s no priority clause in the advanced purchase agreement.”
The shortfall in planned deliveries of the AstraZeneca vaccine is coming at the same time as a slowdown in the distribution of Pfizer-BioNTech shots as Pfizer upgrades production facilities at a plant in Belgium.
“There are a lot of emotions running in this process right now, and I can understand it: People want vaccine,” Soriot said. “I want the vaccine too, I want it today. But, at the end of the day, it’s a complicated process.″
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.