People 75 and older and essential workers next in line for vaccine, panel says

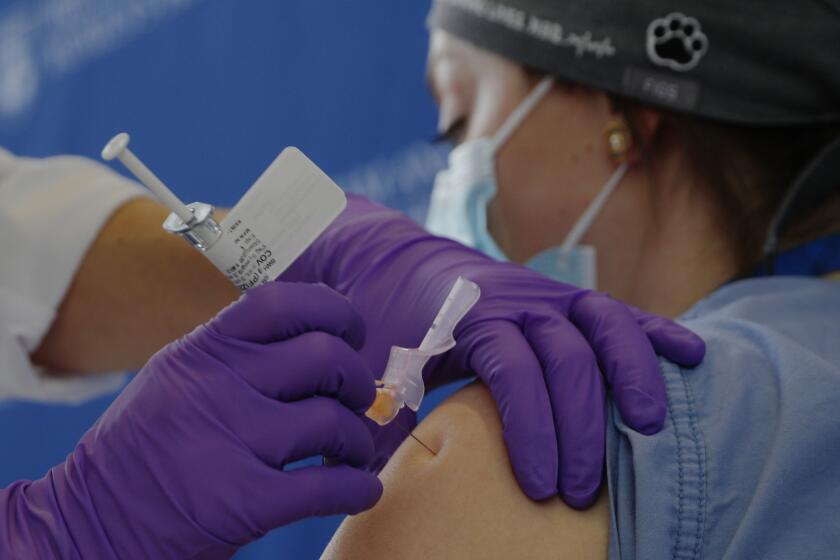
OLIVE BRANCH, Miss. — A federal advisory panel put people 75 and older and essential workers including firefighters, teachers and grocery store workers next in line for COVID-19 shots as a second vaccine began rolling out Sunday to hospitals.
The two developments come as the nation seeks to ramp up a vaccination program, begun just in the last week, that has given initial shots to about 556,000 Americans, according to the Centers for Disease Control and Prevention.
The COVID-19 vaccine developed by Pfizer Inc. and Germany’s BioNTech already is being distributed, and regulators last week gave approval to one from Moderna Inc.
Americans waiting their turn for a COVID-19 vaccine may not be pleased to see who’s in line ahead of them. Does a prisoner or smoker deserve it more?
Earlier this month, the Advisory Committee on Immunization Practices said healthcare workers and nursing home residents — about 24 million people — should be first in line for the vaccines.
Sunday’s vote by the panel concerned who should be next, and by a vote of 13-1, it decided that next in line should be people 75 and older, who number about 20 million, as well as certain frontline workers, who total about 30 million. Those essential workers include firefighters and police; teachers and school staff; those working in food, agricultural and manufacturing sectors; corrections workers; U.S. Postal Service employees; public transit workers; and grocery store workers.
They are considered at very high risk of infection because their jobs are critical and require them to be in regular contact with other people.
It’s not clear how long it will take to vaccinate those groups. Vaccine doses have come out slower than was earlier projected. But at the same time, some experts note that not everyone who is recommended to get vaccinated may choose to get a shot.
The committee also voted that behind those groups should be people ages 65 to 74, numbering about 30 million; those ages 16 to 64 with certain medical conditions such as obesity and cancer who are at higher risk if they become infected with COVID-19, numbering as many as 110 million; and a tier of other essential workers. This group of as many as 57 million includes a wide category of food service and utility workers but also those in legal and financial jobs and the media.
The expert panel’s recommendation next goes to the CDC director and to states as guidance to put together vaccination programs. CDC directors have almost always signed off on committee recommendations. No matter what the CDC says, there will be differences from state to state, because various health departments have different ideas about who should be closer to the front of the line.
Federal officials expect that vaccine doses will be limited for several months. CDC officials say up to 20 million are projected to start getting shots this month, another 30 million next month, and 50 million in February. That’s 100 million people, out of a population of more than 330 million.
Pfizer’s shots were first shipped out a week ago and started being used the next day, kicking off the nation’s biggest vaccination drive.
Public health experts say the shots — and others in the pipeline — are the only way to stop a virus that has been spreading wildly. Nationwide, more than 219,000 people per day on average test positive for the virus, which has killed over 316,000 in the U.S. and nearly 1.7 million worldwide.
Earlier Sunday, trucks left the Olive Branch, Miss., factory, near Memphis, with the vaccine developed by Moderna and the National Institutes of Health. The much-needed shots are expected to be given starting Monday, just three days after the Food and Drug Administration authorized their emergency rollout.
A group of scientists and experts endorsed the COVID-19 vaccine by Moderna and the National Institutes of Health, leading to distribution in California.
In Louisville, Ken., UPS driver Todd Elble said his vaccine shipment was the “most important load that I’ve hauled” in a 37-year career. His parents contracted COVID-19 in November, and his 78-year-old father died. He said the family speculated that his father became infected while traveling on a hunting trip with four other relatives to Wyoming, and some are still sick.
“I’m going to take the vaccine myself. I’m going to be first in line for my father — I’ll tell you that much — and any others that should follow,” he said. “I feel in my heart that everybody should, to help get this stopped.”
He added: “To bring this back, I feel Dad was in the truck with me today.”
Dr. Moncef Slaoui, the chief science advisor to the federal government’s vaccine distribution effort, said on CNN’s “State of the Union” that nearly 8 million doses would be distributed Monday, about 5.9 million of the Moderna vaccine and 2 million of the Pfizer vaccine.
Slaoui also predicted the U.S. would experience “a continuing surge,” with larger numbers of coronavirus cases possible from gatherings for Christmas.
“I think, unfortunately, it will get worse,” he said.
There won’t be enough shots for the general population until spring, so doses will be rationed at least for the next several months. President-elect Joe Biden pledged earlier this month to have 100 million doses distributed in his first 100 days in office, and his surgeon general nominee said Sunday that it was still a realistic goal.
But Vivek Murthy told NBC’s “Meet the Press” it was more realistic to think it might be midsummer or early fall before vaccines were available to the general public, rather than late spring. Murthy said Biden’s team was working toward having the shots available to lower-risk individuals by late spring but doing so required “everything to go exactly on schedule.”
Meanwhile, Trump’s surgeon general, Jerome Adams, defended the administration’s handling of the Pfizer vaccine Sunday, a day after the Army general in charge of getting COVID-19 vaccines across the U.S. apologized Saturday for “miscommunication” with states over the number of doses to be delivered in the early stages of distribution. At least a dozen states reported they would receive a smaller second shipment of the Pfizer vaccine than they had been told previously.
Gen. Gustave Perna told reporters in a telephone briefing that he made mistakes by citing numbers of doses that he believed would be ready. Slaoui said the mistake was assuming vaccines that had been produced were ready for shipment when there was a two-day delay.
“And unless it’s perfectly right, we will not release vaccine doses for usage,” he said. “And, sometimes, there could be small hiccups. There have been none, actually, in manufacturing now. The hiccup was more into the planning.”
But Adams told CBS’ “Face the Nation” that “the numbers are going to go up and down.”
“It absolutely was not poor planning,” he said. “There’s what we plan. There’s what we actually allocate. There’s what’s delivered, and then there’s what’s actually put in people’s arms.”
Adams, who is Black, said he understood that mistrust of the medical community and the vaccine among Blacks “comes from a real place,” the mistreatment of communities of color. He cited the decades-long Tuskegee experiment in Alabama, where Black men with syphilis were not treated so the disease could be studied.
He also said immigrants in the U.S. illegally should not be denied the vaccine because of their legal status because “it’s not ethically right to deny those individuals.”
“I want to reassure people that your information when collected to get your second shot, if you get the Pfizer or Moderna vaccine, will not be used in any way, shape or form to harm you legally,” Adams said. “That is something that I have been assured of.”
Both the Moderna vaccine and the Pfizer-BioNTech shot require two doses several weeks apart. The second dose must be from the same company as the first. Both vaccines appeared safe and strongly protective in large, still unfinished studies.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.