Morocco to use unproven Chinese COVID-19 vaccine to start mass inoculations

RABAT, Morocco — Morocco is gearing up for an ambitious COVID-19 vaccination program, aiming to vaccinate 80% of its adults in an operation starting this month that’s relying initially on a Chinese vaccine that has not yet completed advanced trials to prove it is safe and effective.
The first injections could come within days, a health ministry official told the Associated Press. Facing a public skeptical about the vaccines’ safety and effectiveness, medical experts and health officials have appeared on television in recent weeks to promote the COVID-19 vaccines and encourage Moroccans to get immunized.
While Britain began its vaccination program Tuesday with the Pfizer-BioNTech vaccine and the U.S. and European Union are racing to approve a series of Western-made vaccines, other governments are looking to use vaccines from China and Russia.
The World Health Organization has said new vaccines should first be tested in tens of thousands of people to prove they work and don’t cause worrisome side effects before being rolled out broadly. But the U.N. health agency also says it is up to individual countries to decide whether there is an urgent domestic need to use a vaccine even without such data.
Morocco is battling a resurgence in coronavirus infections, with the number of recorded COVID-19 deaths surpassing 6,000. The North African kingdom is pinning its hopes on two vaccine candidates, one developed by China’s Sinopharm and the other by Britain’s Oxford University and AstraZeneca.
The Sinopharm vaccine has been approved for emergency use in a few countries, but the company is still conducting late-stage clinical trials in 10 countries. The AstraZeneca vaccine is still in advanced trials in countries including Britain and the U.S. and hasn’t been approved yet.
Britain becomes the first Western nation to start vaccinating residents against COVID-19, launching a mass inoculation program that will take months.
Morocco’s government seeks to vaccinate 80% of its adults, or 25 million people, as soon as the vaccines are approved by domestic regulators. Priority will go to medical staff and other front-line workers, as well as the elderly.
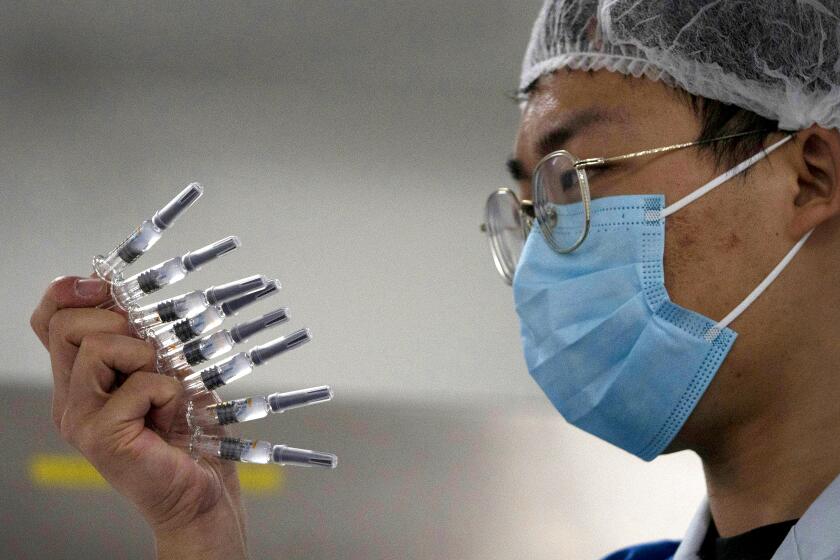
It will start with the Sinopharm vaccine, which was tested on 600 Moroccans as part of clinical trials this autumn. Morocco has ordered 10 million doses of the vaccine.
The initial deliveries will come from China, but Morocco also plans to produce the vaccine locally, Abdelhakim Yahyan, a senior official at the Ministry of Health, told the state-owned news agency MAP.
Health Minister Khalid Ait Taleb said Morocco is seeking vaccines from several sources because COVID-19 vaccines are a scarce commodity and a single manufacturer’s production capacity is too limited to meet the needs of the whole world.
China has already injected hundreds of thousands of people with COVID-19 vaccines. Vaccines that have not yet completed clinical trials.
In the Moroccan trial of the Sinopharm vaccine, carried out in Casablanca and the capital, Rabat, from August through November, healthy volunteers received two separate doses of the vaccine. In the advanced trial, volunteers either received the vaccine or a placebo. According to the health minister, early results have proven the vaccine to be “safe and effective,” with no severe side effects reported.
However, some Moroccans have taken to social media to question the safety of the vaccine, with some noting that China was the original epicenter of the pandemic and questioning how effective it will be.
Sinopharm’s shot relies on a tested technology, using a killed virus to deliver the vaccine, similar to how polio immunizations are made. Leading Western competitors, such as the vaccine developed by Oxford and AstraZeneca, use newer, less-proven technology to target the coronavirus’ spike protein.
In China, the state-owned Sinopharm subsidiary CNBG has given the vaccine to 350,000 people outside its clinical trials, a CNBG executive has said.
Critics in Morocco have also expressed concerns that citizens might be forced to take the vaccine, but the health minister insisted that COVID-19 vaccinations will not be mandatory and will be free.
Prime Minister Saad-Eddine El Othmani has sought to reassure the hesitant about the robustness of the country’s regulatory process for vaccine approval, saying that no corners have been cut in making sure the Chinese vaccine is safe to administer.
Morocco’s mass immunization operation will entail 2,888 vaccination stations and the deployment of mobile units to vaccinate people at factories, offices, campuses and prisons. The health ministry said it would mobilize more than 12,000 health professionals as well as the military to ensure rapid distribution.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.