COVID-19 shots unlikely to prompt rare inflammation in kids
COVID-19 vaccines are unlikely to trigger a rare inflammatory condition linked to coronavirus infection in children, according to an analysis of U.S. government data published Tuesday.
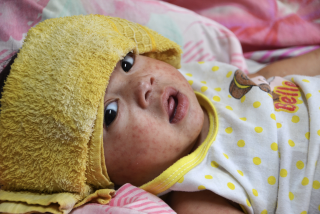
The condition, formally known as multisystem inflammatory syndrome in children, involves fever plus symptoms affecting at least two organs and often includes stomach pain, skin rash or bloodshot eyes. It’s a rare complication in kids who have had coronavirus infections, and very rarely affects adults. The condition often leads to hospitalization, but most patients recover.
First reported in the United Kingdom in early 2020, it is sometimes mistaken for Kawasaki disease, which can cause swelling and heart problems. Since February 2020, more than 6,800 cases have been reported in the U.S., according to the Centers for Disease Control and Prevention.
As part of COVID-19 vaccine safety monitoring, the CDC and U.S. Food and Drug Administration added the condition to a list of several potential adverse events of special interest. A few cases reported in people with no detectable evidence of coronavirus infection prompted researchers at the CDC and elsewhere to undertake the new analysis, which was published Tuesday in The Lancet Child & Adolescent Health.
The possibility that the vaccines could somehow prompt the condition is only theoretical and the analysis found no evidence that it did, said co-author Dr. Buddy Creech, a Vanderbilt University pediatric infectious disease specialist who is leading a study of Moderna shots in children.
“We don’t know what the exact contribution of the vaccine to these illnesses is,” Creech said. “Vaccine alone in absence of a preceding infection appears not to be a substantial trigger.”
New U.S. research shows most kids with a serious inflammatory illness called MIS-C linked to the coronavirus were symptom-free or had mild infections.
The analysis involved surveillance data for the first nine months of COVID-19 vaccination in the U.S., from December 2020 through August 2021. During that time, the FDA authorized Pfizer’s COVID-19 shots for people ages 16 and up; expanded that in May to adolescents ages 12 through 15; and authorized Moderna and Johnson & Johnson shots for ages 18 and up.
More than 21 million people aged 12 to 20 received at least one vaccine dose during that time. Twenty-one of them developed the inflammatory condition afterward. All had received Pfizer shots, the analysis found.
Fifteen of the 21 had laboratory evidence of a previous coronavirus infection that could have triggered the condition.
The remaining six had no evidence of a previous infection, but the researchers said they could not conclude definitively that they’d never caught the coronavirus or some other infection that could have led to the inflammatory condition. Kids with the coronavirus often have no symptoms and many never get tested.
The results suggest that the inflammatory condition may occur after vaccination in 1 in 1 million children who have had the coronavirus, and in 1 in 3 million who have no detectable evidence of previous coronavirus infection.
Suspicion, misinformation and other factors result in what authorities say are alarmingly low COVID vaccination rates in U.S. children ages 5 to 11.
Most kids who were infected don’t develop the post-infection illness, but it is estimated to happen at a significantly higher rate than both of those post-vaccination figures. In April to June 2020, the rate was 200 cases per million in unvaccinated, infected people in the U.S. between the ages of 12 and 20.
“Their findings overall are quite reassuring,” Dr. Mary Beth Son of Boston Children’s Hospital wrote in a commentary accompanying the study.
Dr. Adam Ratner, a pediatrician-scientist at New York University Langone Health, said the results show that chances are “super rare” for the shots to prompt an immune response that could lead to the inflammatory condition. By contrast, there’s strong evidence that vaccination protects kids from getting COVID-19 as well as the condition, Ratner said.