Johnson & Johnson’s one-shot COVID-19 vaccine grants promising response in early study
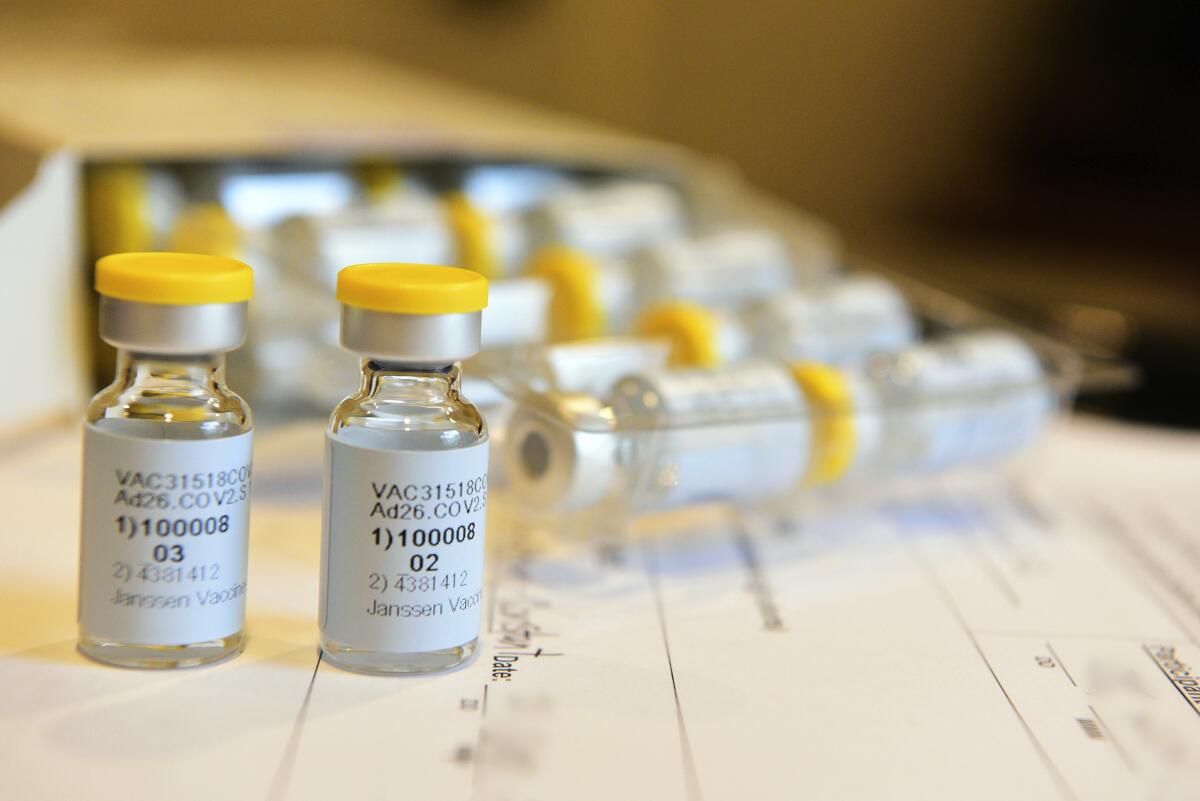
Johnson & Johnson’s experimental one-shot COVID-19 vaccine generated a long-lasting immune response in an early safety study, giving a glimpse at how it will perform in the real world as the company inches closer to approaching U.S. regulators for clearance.
More than 90% of participants made immune proteins, called neutralizing antibodies, within 29 days after receiving the shot, according to the report, and participants formed the antibodies within 57 days. The immune response lasted for the full 71 days of the trial.
“Looking at the antibodies, there should be good hope and good reason that the vaccine will work” in the company’s late-stage clinical trials, Johnson & Johnson Chief Scientific Officer Paul Stoffels said in an interview Tuesday. The company is expected to report results from those late-stage trials soon.
The one-shot vaccine generates more neutralizing antibodies than a single dose of other COVID-19 vaccines, all of which are two-shot regimens. And when compared with two shots of these rivals, the response to Johnson & Johnson’s single shot is in the same range, Stoffels said.
Interim results from a trial of participants 18 and older were published Wednesday in the New England Journal of Medicine. The data expanded on more limited findings that Johnson & Johnson first published in September.
The company’s progress is being closely watched by top infectious-disease experts because its vaccine has the potential to become the first that can protect people after just one shot, making mass-vaccination campaigns much easier. The company expects to get definitive efficacy data from a final-stage study by early next month, potentially leading to regulatory authorization by March.
Doubts surrounding a homegrown vaccine are among the challenges facing India’s effort to vaccinate 300 million people in six months.
The U.S. has granted emergency-use authorizations to two vaccines, one developed by Pfizer and its German partner, BioNTech, and the other by Moderna. Both employ a technology called messenger RNA that has never been used before in an approved product, and each showed more than 90% efficacy.
U.S. government officials had earlier said any vaccine with greater than 50% efficacy would be considered a success. Based on that guidance, Johnson & Johnson aimed for 60% effectiveness, Stoffels said, but “we hoped and we planned for 70%.”
The Johnson & Johnson vaccine candidate is made from a cold virus, called an adenovirus, that’s modified to make copies of the coronavirus’ spike protein, which the pathogen uses to enter cells. Although the altered virus can’t replicate in humans, it induces an immune response that prepares the body for an actual coronavirus infection.
Within weeks, Johnson & Johnson will learn how its vaccine performed in a late-stage trial of 45,000 volunteers. Stoffels now thinks it has the potential to be even more than 70% effective, based on the early-stage findings and other factors.
Health experts decry the chaotic rollout of the vaccine, but government says progress is on the way.
Another advantage to Johnson & Johnson’s shot is that it can be stored at refrigerator temperatures for three months.
The study released Wednesday also found that a second dose of Johnson & Johnson’s shot, administered two months later, led to a threefold increase in neutralizing antibodies. Stoffels called that positive news, and said the company is still evaluating how long immunity from a single shot will last and whether higher antibody levels will be needed to combat new strains of the coronavirus.