Goodbye, nasal swabs? Saliva tests can detect coronavirus infection, studies show
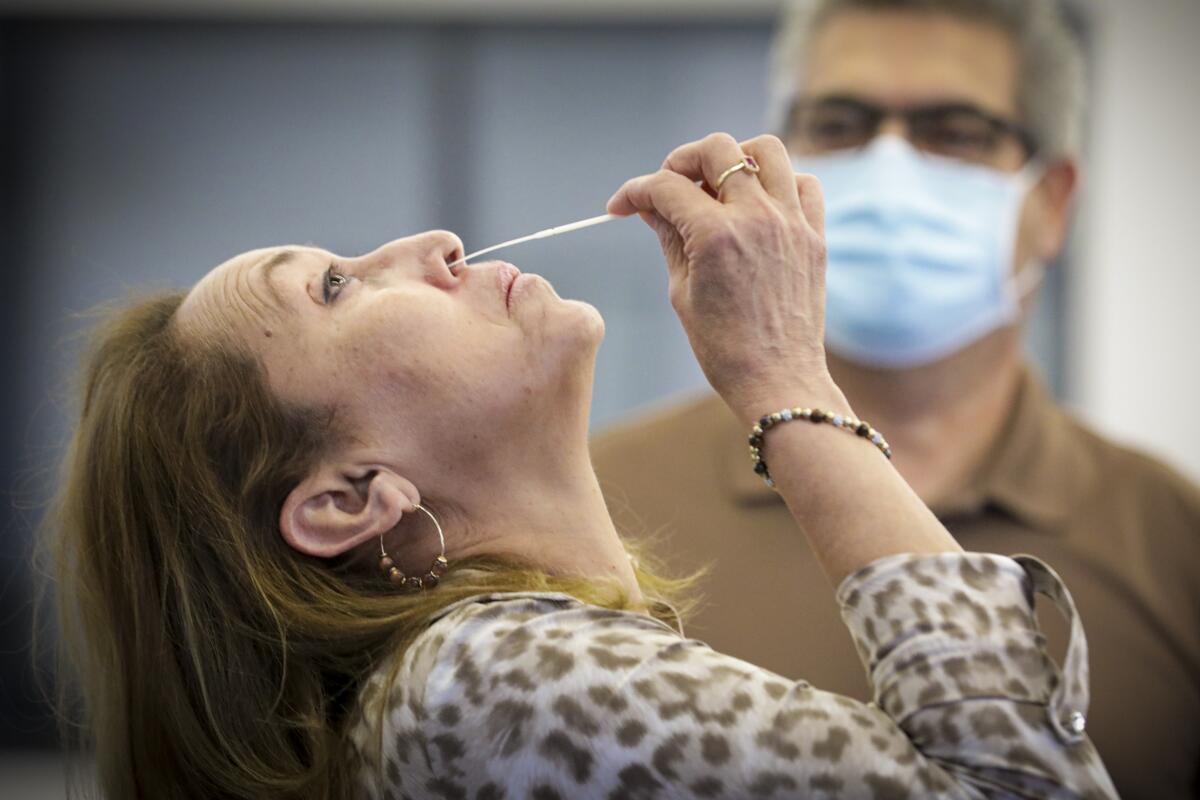
If there’s one thing we can safely predict about the COVID-19 pandemic, it’s that plenty of coronavirus tests lie in our future. Luckily, researchers have some good news on that score.
Two new studies have found that tests that look for the virus in samples of saliva are about as reliable as tests that require a sample from the back of the nose.
That’s sure to be a welcome development to anyone who would rather avoid the discomfort of having a long, stiff swab inserted so far back into their nasal cavity that it feels like it’s tickling their brain.
But it’s not the only benefit. Pretty much anyone can administer a saliva-based test, so there’s no need for a trip to a testing center. It also frees up the time of medical personnel and spares them potential exposure to the virus.
In one of the new studies, a team from Yale identified 70 hospital patients with COVID-19 whose infections had been confirmed with the traditional nasopharyngeal swabs. Each time a healthcare worker carried out additional nasal swab tests, the researchers asked the patients to give themselves a saliva test as well.
The saliva tests did a better job of detecting the virus formally known as SARS-CoV-2, the researchers found. In the first five days after diagnosis, 81% of the saliva tests came back positive, compared with 71% of the nasopharyngeal tests. A similar gap remained through the 10 day after diagnosis.
In addition, the researchers detected more copies of the virus’ genetic material in patients’ saliva than in the samples taken from the back of their nasal cavities.
The incidence of injuries attributed to domestic partner violence rose sharply after the coronavirus outbreak began, and those injuries were more serious.
To see how the tests stacked up among people with asymptomatic infections, the researchers recruited 495 healthcare workers with no signs of COVID-19 and gave them the saliva test. Thirteen of the tests came back positive.
Among those 13 people, nine had given themselves nasal swabs on the same day, and only two of those tests came back positive. However, all 13 of the saliva tests were later confirmed by additional nasopharyngeal tests.
The results were reported Friday in the New England Journal of Medicine.
“Given the growing need for testing, our findings provide support for the potential of saliva specimens in the diagnosis of SARS-CoV-2 infection,” the Yale team wrote.
In the second study, researchers from Canada recruited nearly 2,000 people with either mild symptoms consistent with COVID-19 or who had no symptoms but were at high risk of infection. The study design was meant to simulate the conditions of mass screening, the authors wrote.
Participants submitted to a standard nasal swab test and also collected their own saliva samples. Of the 1,939 pairs of tests, 34 came back positive for coronavirus infection. There were also 14 cases where the virus was detected in the saliva sample but not the nasal sample, and 22 cases where the reverse was true.
These results were published Friday in Annals of Internal Medicine.
One of the hallmarks of the COVID-19 pandemic in the United States is that it disproportionately strikes people of color. But it doesn’t have to be that way.
Although the nasal swab test detected more infections than the saliva test, the latter performed well enough to earn consideration as a screening tool, wrote the team from the University of Ottawa, Dalhousie University and Canada’s National Microbiology Laboratory.
“Saliva testing presents potential advantages,” the researchers wrote. “Collection does not require trained staff or personal protective equipment, can be done outside testing centers, and may be better tolerated in challenging or pediatric populations.”
The Yale team noted some of those same benefits and added a few more. Saliva tests eliminate the need for healthcare workers to come into contact with people who might be infected, reducing transmission risk. Being able to conduct a test without medical personnel on hand also removes a “major testing bottleneck,” the team wrote.
Dr. Stephen Hahn, the commissioner of the U.S. Food and Drug Administration, has also noted that the saliva tests can allow screening to proceed even when the chemical reagents needed for nasal swab tests are in short supply.
At least five saliva tests have received emergency use authorization from the FDA, including one developed at Yale.









