U.S. pumps $1.6 billion into Novavax’s COVID vaccine development

Novavax Inc., one of the front-runners in the race to develop a COVID-19 vaccine, will receive $1.6 billion from the U.S. government, the biggest contribution yet from the Operation Warp Speed program.
The funds will allow the company to conduct advanced human studies and establish manufacturing to deliver 100 million doses as soon as late 2020, Gaithersburg, Md.-based Novavax said in a statement.
Shares of Novavax rose as much as 41%, the highest since September 2016, in Tuesday trading.
Novavax is among companies striving to develop an inoculation against the novel coronavirus that’s spreading quickly in countries including the U.S., India and Mexico. President Trump’s Warp Speed program has backed efforts at a number of companies, including Johnson & Johnson, Merck & Co., Pfizer Inc., Moderna Inc. and AstraZeneca Plc, to get doses as early as possible.
Operation Warp Speed seeks to compress a process that is typically years long into a matter of months. The drive is being led by Gen. Gustave Perna, who directs the U.S. Army Materiel Command, and former GlaxoSmithKline Plc executive Moncef Slaoui.
Spike protein
The funds will help Novavax begin a final-stage study of its vaccine candidate as early as this fall, with as many as 30,000 subjects, according to the statement.
In May, biotech company earlier secured as much as $388 million from the Coalition for Epidemic Preparedness Innovations, the single largest contribution from the organization at the time. The company’s vaccine candidate is meant to provoke the production of antibodies that block the “spike” protein the coronavirus uses to infect host cells.
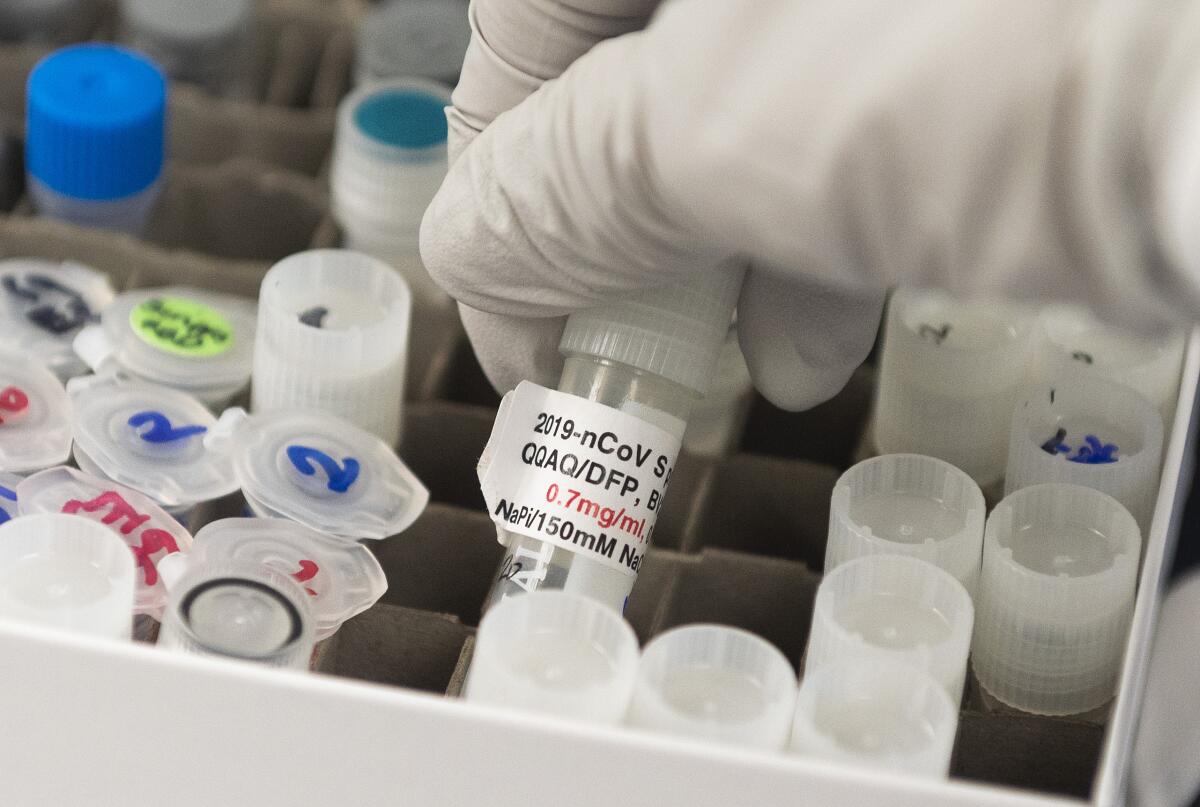
Separately, Regeneron Pharmaceuticals Inc. secured a $450-million award from Operation Warp Speed under the auspices of the U.S. Biomedical Advanced Research and Development Authority, or BARDA. The Tarrytown, N.Y.-based company will use the funds to scale up production of an antibody cocktail to prevent infection.
Analysts are expecting results from Regeneron’s antibody program sometime in the third quarter. Novavax, which has yet to commercialize a medicine or vaccine, plans to report a first look at its results in patients later this month.
Drug companies and university researchers are investigating more than 140 experimental inoculations, according to the World Health Organization. Moderna, Pfizer and the University of Oxford, working with AstraZeneca, are among the companies and institutions that have started studies of their vaccines in healthy patients.