NIH and top scientists call for moratorium on gene-edited babies
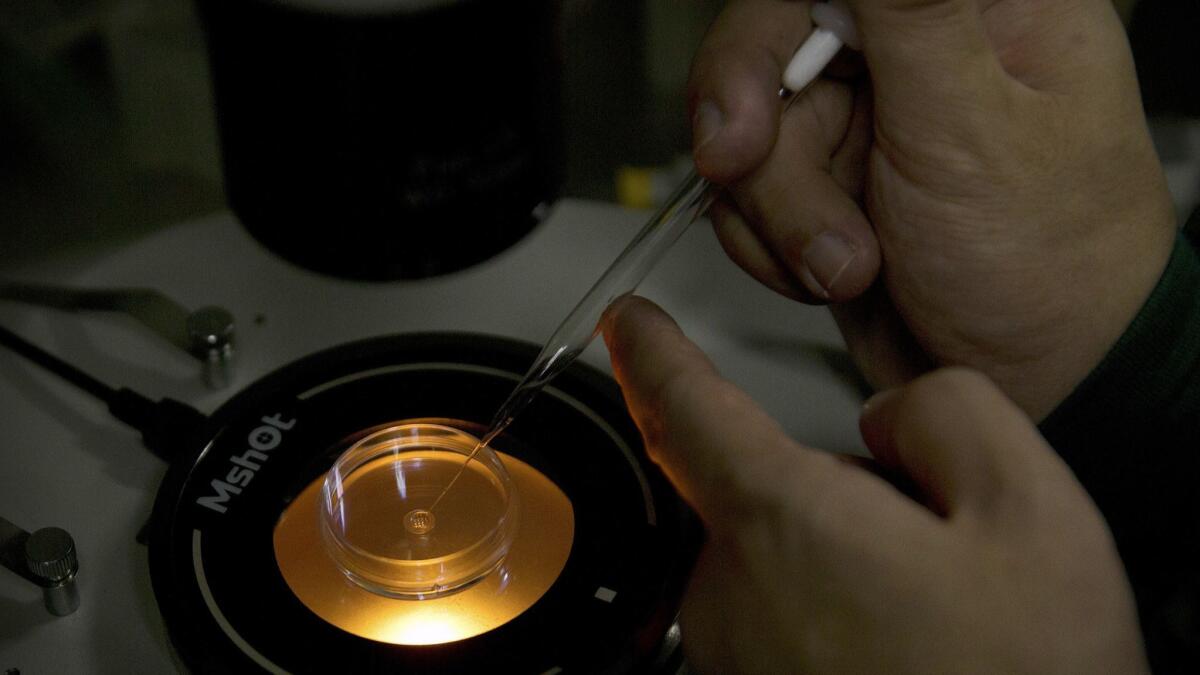
Scientists and ethicists from seven nations on Wednesday called for a moratorium on gene-editing experiments designed to alter heritable traits in human babies.
It’s the latest alarm sounded by researchers who have been both excited and unnerved by the powerful genetic engineering technique known as CRISPR, which can potentially prevent congenital diseases but also could lead to permanent changes in human DNA and possibly create a market for enhanced, augmented offspring, sometimes called “designer babies.”
The call for the moratorium, published as a commentary in the journal Nature, came in direct response to the actions of a Chinese researcher who, disregarding a global consensus on the ethical boundaries of gene editing, altered embryos that were implanted and carried to term, resulting in the live birth of twin babies.
The Chinese researcher, He Jiankui, said his experiment was intended to alter a gene to make the babies resistant to infection with HIV. He said he knew he would be criticized but defended it as an ethical form of gene therapy and not a steppingstone to making cosmetic genetic alterations.
Why geneticists say it’s wrong to edit the DNA of embryos to protect them against HIV »
But the scientific community was outraged, condemning He’s actions as “rogue human experimentation.” The new call for a moratorium is an acknowledgment that the many warnings emerging from conferences on the ethics of gene editing have not been sufficiently clear and emphatic, and, in the case of the Chinese twins, have failed to prevent an ethical violation.
The authors of the Nature paper include two of the primary inventors of the CRISPR system — Feng Zhang of the Broad Institute of MIT and Harvard and Emmanuelle Charpentier of the Max Planck Unit for the Science of Pathogens in Berlin. In addition to calling for a moratorium, the authors argue for the creation of an international governing body that would oversee the application of the technology.
Separately on Wednesday, Dr. Francis Collins, director of the National Institutes of Health, issued a statement supporting the call for a moratorium and a governing body. In an interview, he made clear that this is the U.S. government position, discussed and cleared at the highest levels.
“What we’re talking about here is one of the most fundamental moments of decision about the application of science to something of enormous societal consequence. Are we going to cross the line toward redesigning ourselves?” Collins said.
The new Nature paper does not call for a permanent ban on gene editing of heritable traits. It’s a call for a temporary stop, with no firm expiration of the moratorium. It focuses specifically on experiments involving sperm, eggs and embryos — also known as germ line cells — that are designed to result in pregnancy.
The moratorium would not cover laboratory research not intended to result in a birth nor gene editing for therapeutic purposes in a patient’s nongerm-line cells — called somatic cells — because those changes wouldn’t be passed down from generation to generation.
The authors of the Nature paper call for an “international framework” supported by a coordinating body that could either be fully independent or part of the World Health Organization. The authors envision voluntary compliance by individual nations that would retain sovereignty over their scientific enterprises.
One name is notably absent from the list of authors of the Nature paper, that of CRISPR pioneer Jennifer Doudna of UC Berkeley. Doudna is a powerful voice on this issue, having not only invented much of the CRISPR technology but also warning early on that it could be used for malign purposes.
Doudna said she declined a request by Zhang to join this new call for a moratorium and new governing body. She said she will continue, instead, to work with the national academies in the United States, the United Kingdom and China.
“My feeling is, this is effectively just rehashing what’s been going on for several years,” Doudna said.
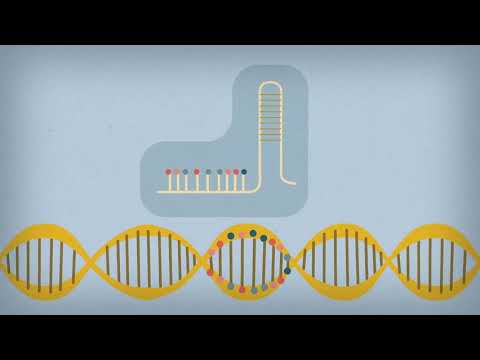
CRISPR provides a way to find and alter specific DNA sequences.
The consensus among scientists and ethicists has been that CRISPR and other gene-editing techniques can have many desirable applications. That would include research on cells, including human embryos, so long as the modified cells weren’t used to establish a pregnancy.
There is no objection to using gene editing in somatic cells to treat an individual patient in a way that does not pass along those changes. One example: editing the genes in blood cells to relieve sickle-cell anemia.
But the consensus is that there’s a bright line: No one should edit genes in a way that could become a permanent trait of the human species unless there is broad agreement that such a modification is safe, necessary and ethical.
The United States has laws that prevent this kind of germ-line editing. Legislation requires that such experiments receive approval from the Food and Drug Administration, which in turn is prohibited by law from evaluating such proposals. The Nature paper states that about 30 nations have laws that directly or indirectly prevent this kind of genetic engineering.
Eric Lander, lead author of the commentary in Nature and the head of the Broad Institute of MIT and Harvard, said the effort to keep CRISPR under international guidance could be a template for handling powerful new technologies more generally.
“I think it raises the question, how do we govern complex technology,” Lander said. “Powerful technologies, we just increasingly see they have upsides and downsides. We can’t just throw up our hands and say there’s no way to stop it. There is a way to guide it.”
Lander said that after the revelation of He’s experiment in China, he and Zhang discussed the need to make a new call for some way to halt rogue application of the technology. They recruited other prominent researchers in the field and collaborated on the article.
CRISPR, which stands for clustered regularly interspaced short palindromic repeats, leverages a natural bacterial system that targets viruses that invade a cell. It has been described as molecular scissors. Technicians can use this system to alter an organism’s genome, for example by deleting a genetic mutation associated with a disease.
Invented early in this decade, this type of gene editing has become more precise, with fewer off-target edits. Some clinical trials with human patients are underway, but they do not involve germ-line cells.
The authors of the Nature commentary make a distinction between genetic “correction,” for therapeutic medical purposes, and genetic “enhancement,” which might include “incorporating new instructions into a person’s genome to enhance, say, their memory or muscles, or even to confer entirely new biological functions, such as the ability to see infrared light or break down certain toxins.”
“I think it’s a very powerful technology that has a lot of potential to improve our lives, improve our health, improve our environment, improve our agriculture,” Zhang said of CRISPR. But he said that, like any powerful technology, “we can get ahead of ourselves,” and he raised the specter of what some people have called “designer babies” 3 genetic modifications that are meant to enhance or augment offspring and have no medical necessity.
“You can imagine a situation where parents will feel pressure to edit their children because other parents are,” Zhang said. “It could further exacerbate inequality. It could create a total mess in society.”






