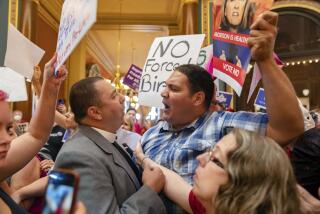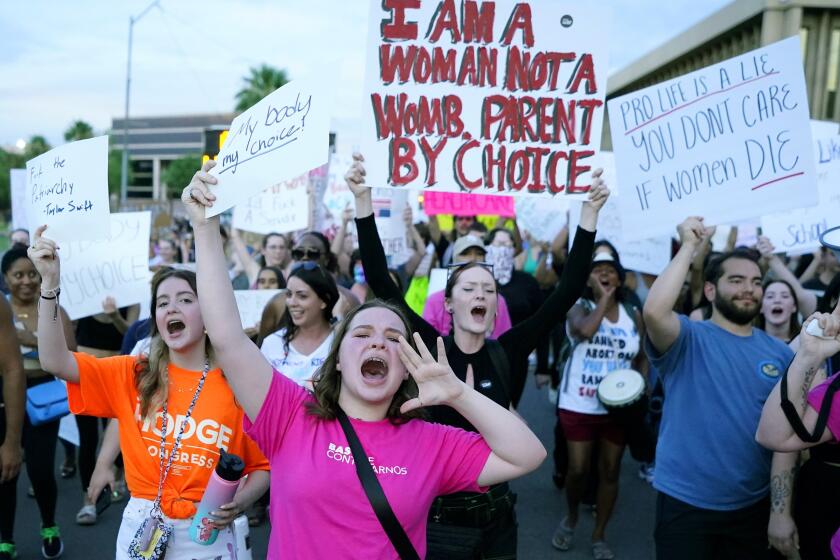Supreme Court agrees to rule on FDA’s approval of abortion pills sent by mail
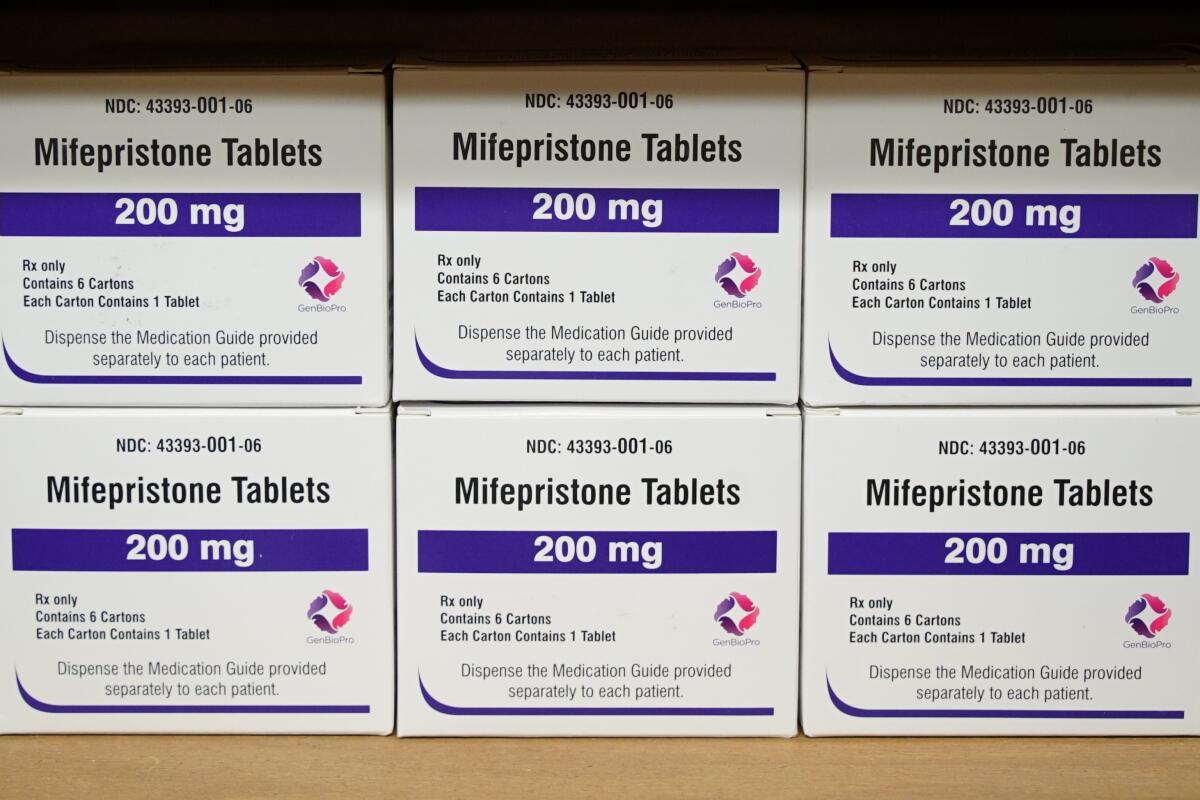
WASHINGTON — The Supreme Court said Wednesday it will decide whether to put stricter limits on abortion pills that are now the most common method for ending early pregnancies.
The justices voted to hear the Biden administration’s appeal and reconsider rulings by conservative judges in Texas who disagreed with the Food and Drug Administration’s view that mifepristone is safe and effective and may be dispensed widely.
At issue are FDA regulations in 2016 and 2021 that extended the time for using the pills from seven weeks to 10 weeks of pregnancy and allowed for dispensing the medication without requiring one or more visits to a doctor’s office. Currently the pills may be dispensed through a pharmacy or sent through the mail.
The justices said they had voted to hear appeals from the FDA and Danco Laboratories, which manufactures the pills, and they turned down an appeal filed by the antiabortion activists who sought to have the pills taken off the market.
That is a good sign for the administration lawyers, because it shows the justices are not willing to even hear a broad argument for taking the pills off the market.
Since the drugs were first approved by the FDA in 2000, they have been used by more than 5 million women in this country and even more around the world, government lawyers said.
The FDA case will be heard in the spring, and it is the most significant abortion case since the court’s conservative majority struck down the constitutional right to abortion in 2022.
If the court were to rule for antiabortion advocates who sued in Texas, it could limit the legal use of abortion medication to seven weeks of a pregnancy and require patients to make one or more visits to a doctor.
“The future of telehealth for medication abortion now hangs in the balance,” said Dana Northcraft, director of the Reproductive Health Initiative for Telehealth Equity & Solutions. “Telehealth for medication abortion is safe and effective and helps people overcome barriers to care, whether it be long travel distances or getting time off from work or school.”
It’s not clear, however, whether a Supreme Court ruling upholding the FDA’s regulations will help many women in states like Texas where abortion is illegal.
“It’s a complicated question,” said Greer Donley, a law professor at the University of Pittsburgh.
In one sense, a ruling in favor of the FDA “will have no impact on people in states like Texas because no one is providing abortion there, with medication or otherwise. However, blue state shield providers are shipping abortion medication, including mifepristone, into red states.”
Antiabortion advocates say they fear a Supreme Court ruling upholding the FDA would lead to a “national mail-order abortion scheme.”
In April, the administration won a temporary victory when the justices voted to block the rulings of a west Texas judge and the 5th Circuit Court of Appeals. Justices Clarence Thomas and Samuel A. Alito Jr. dissented.
In her appeal, Solicitor Gen. Elizabeth B. Prelogar argued the antiabortion activists who brought the original case in Amarillo, Texas, had no standing to sue, and she said the Texas judges had no basis for overturning the FDA’s regulations.
Government lawyers argued that the antiabortion doctors who sued do not perform abortions and do not prescribe the abortion medication for their patients. Therefore, the government asserts, they cannot claim to be personally harmed simply because other doctors disagree with them.
“This court should not allow the speculative fears of a handful of doctors to deprive patients throughout the country of an essential medication ... or denied treatment for miscarriage or other early pregnancy loss because of [their] hypothetical fears or personal beliefs,” the American College of Obstetricians and Gynecologists and the American Medical Assn. said in urging the court to uphold use of the abortion medication.
Prelogar said the Texas rulings at issue “mark the first time any court has restricted access to an FDA-approved drug based on disagreement with FDA’s expert judgment about the conditions required to assure that drug’s safe use — much less done so after those conditions had been in effect for years.”
While the district judge in Amarillo would have ordered the drugs to be taken off the market, the 5th Circuit judges agreed it was too late to challenge the FDA’s decisions in 2000 to approve the use of mifepristone. But the 5th Circuit rejected regulatory updates in 2016 and 2021 that allowed for dispensing the drugs through pharmacies and the mail.
She said the courts should not veto “a scientific judgment FDA has maintained across multiple administrations” and impose “unnecessary restrictions on the distribution of a drug that has been used safely by millions of Americans over more than two decades.”
Lawyers for the Alliance Defending Freedom, which sued to halt use of the abortion pills, described the 5th Circuit’s approach as “a modest decision [that] merely restores the common-sense safeguards under which millions of woman have taken chemical abortion drugs.”
More to Read
Get the L.A. Times Politics newsletter
Deeply reported insights into legislation, politics and policy from Sacramento, Washington and beyond. In your inbox three times per week.
You may occasionally receive promotional content from the Los Angeles Times.