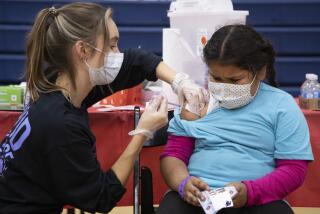
COVID vaccines may soon be available for kids under 5. What Californians need to know
Federal regulators are moving toward authorizing COVID-19 vaccines for young children, meaning the littlest Californians could begin rolling up their sleeves by early next week.
The Food and Drug Administration on Friday authorized offering the shots to children under 5 — the last major age group not yet eligible for vaccination.
The matter is now in the hands of the U.S. Centers for Disease Control and Prevention, which could give its blessing as soon as this weekend.
“Many parents, caregivers and clinicians have been waiting for a vaccine for younger children, and this action will help protect those down to 6 months of age,” FDA Commissioner Dr. Robert Califf said in a statement.
“As we have seen with older age groups, we expect that the vaccines for younger children will provide protection from the most severe outcomes of COVID-19, such as hospitalization and death.”
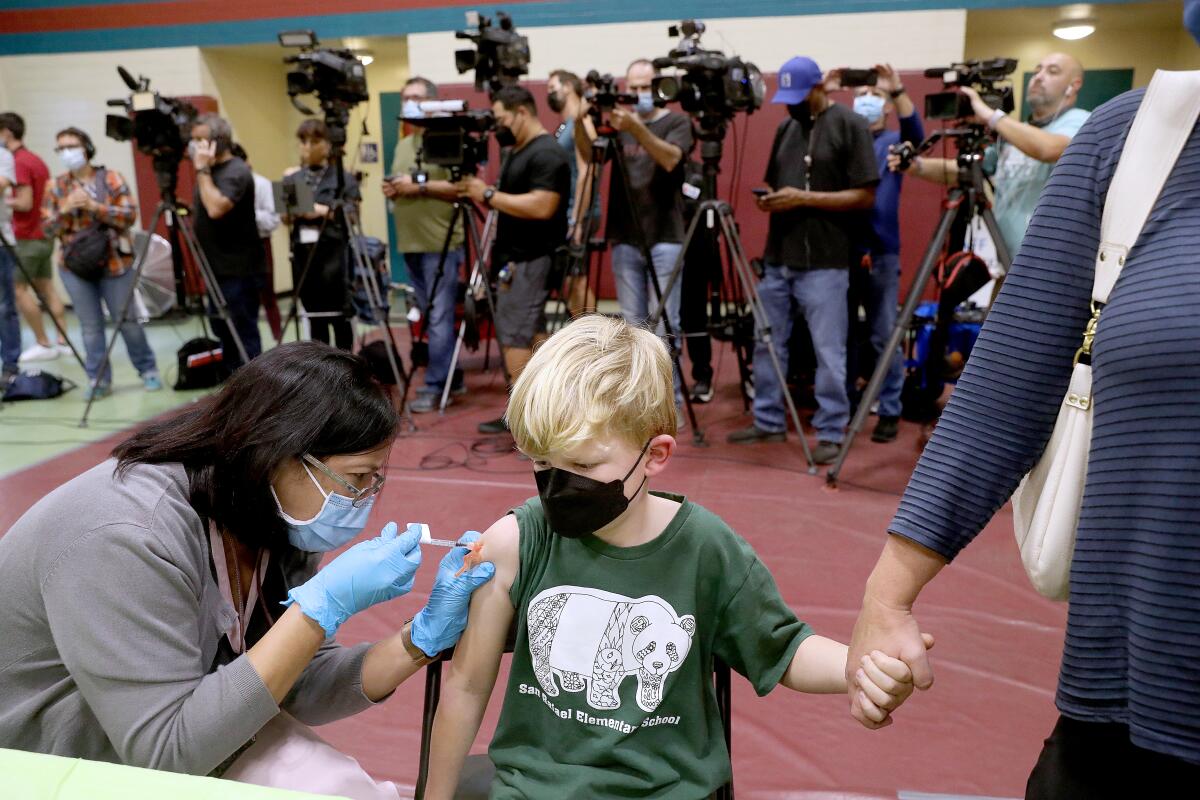
U.S. youngsters under 5 will be eligible for the shots as soon as next week, about 1 1/2 years after the vaccines first became available in the U.S. for adults.
Already, California health officials are positioning themselves to begin doling out doses as soon as possible.
Here’s what you need to know:
What’s changing?
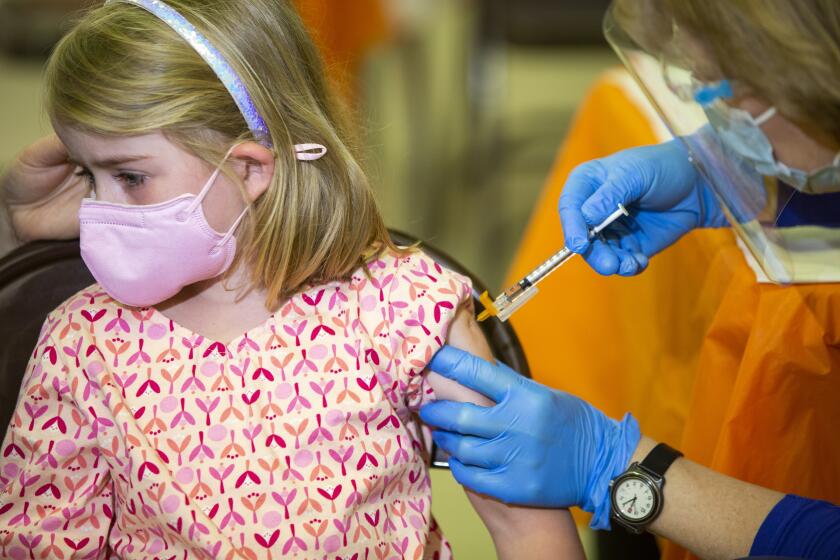
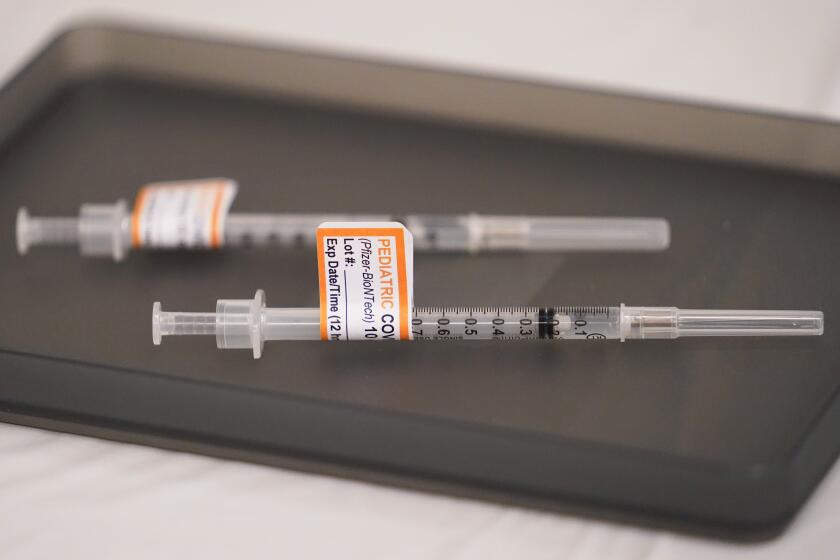
The FDA’s emergency-use authorization would allow children as young as 6 months old to receive either the Pfizer-BioNTech or Moderna vaccine.
Pfizer-BioNTech is already available for those who are at least 5 years old. Moderna has, to this point, been an adults-only vaccine.
What is the vaccination process?
The Moderna vaccine would be administered as a two-dose course for the youngest children, with the shots given one month apart. Each shot is a quarter of the typical adult dose.
Three Pfizer shots — each one-tenth the dose given to adults — are needed for the youngest children.
The initial two should be given three weeks apart, with the third following at least eight weeks after, according to the FDA.
Biden administration says children under 5 may be able to get their first COVID-19 vaccination dose as soon as June 21, if the FDA approves as expected.
When will the shots be available?
CDC vaccine advisors will discuss expanding pediatric vaccine access Friday and Saturday and are scheduled to vote on the matter Saturday.
After that, CDC officials will decide whether to formally recommend them.
The Western States Scientific Safety Review Workgroup — a coalition of public health experts from California, Nevada, Oregon and Washington — will also conduct its own review.
In a statement, the California Department of Public Health said it “will await further action from the CDC and Western States before making changes to vaccine administration.”
But should all go as planned, health officials say they expect to start this next phase of the vaccination campaign within a matter of days.
Barbara Ferrer, Los Angeles County’s public health director, said she expects those vaccines will be available as soon as Tuesday.
How many doses will there be?
California has already placed orders for pediatric formulations of Pfizer and Moderna and expects to receive an initial shipment of 243,000 doses “by early next week,” the state Department of Public Health wrote this week in response to an inquiry from The Times.
“We are working closely with the state’s local health departments, vaccines for children providers and other providers of pediatric services such as those from healthcare systems to distribute and administer infant/toddler COVID-19 vaccine as quickly as possible,” that statement continued.
An average of about 15,900 new coronavirus infections a day were reported over the last week across California, health officials say.
Where can I make an appointment?
Officials say vaccines for the youngest children will be available at a variety of locations.
More information on sites and appointment openings will be accessible through the state’s online platform, MyTurn.ca.gov.
The California Department of Public Health said it would “monitor appointment wait times in MyTurn.ca.gov and collaborate with our local agency partners to assess demand for vaccinations in this age group and how well the system is keeping up with demand.”
L.A. County “will have over 900 locations prepared to begin administering vaccines to our youngest residents,” and “most of that will start up early next week,” according to Ferrer.
“This would include almost 180 healthcare provider facilities, over 200 pharmacies and over 500 mobile sites and our seven [Department of Public Health] sites,” she told reporters Thursday.
But, she added, “because some of the pharmacy sites are only licensed to vaccinate children 3 and older, parents are encouraged to reach out ahead of time to verify hours and availability.”
More information will also be available at VaccinateLACounty.com.
Should I consider getting my young child vaccinated?
Throughout the pandemic, COVID-19 generally hasn’t hit younger children as hard as other age groups. But officials and experts say kids are not immune to potentially devastating health impacts that vaccinations could help thwart.
According to a report published by the CDC in March, weekly COVID-19 hospitalization rates among children 4 and younger were five times as high during the peak of the first Omicron surge that struck over the fall and winter compared with last summer’s Delta wave.
Another study published by the CDC in April said that COVID-19 hospitalization rates among 5- to 11-year-olds were twice as high among unvaccinated children compared with those who were vaccinated.
Many older California children and teens have already been vaccinated.
According to data compiled by The Times, 67% of those ages 12 to 17 have been fully vaccinated.
However, the same is true of just 35% of 5- to 11-year-olds.
In authorizing the shots for younger children, the FDA “determined that the known and potential benefits of the Moderna and Pfizer-BioNTech COVID-19 vaccines outweigh the known and potential risks in the pediatric populations authorized for use for each vaccine,” according to a statement from the agency.
“Those trusted with the care of children can have confidence in the safety and effectiveness of these COVID-19 vaccines and can be assured that the agency was thorough in its evaluation of the data,” Califf said.
More to Read
Sign up for Essential California
The most important California stories and recommendations in your inbox every morning.
You may occasionally receive promotional content from the Los Angeles Times.