AstraZeneca secures orders for 400 million doses of a potential coronavirus vaccine
LONDON — Drug maker AstraZeneca said Thursday that it had secured orders for at least 400 million doses of an as-yet-unproven COVID-19 vaccine being developed with Oxford University and that it would begin delivering them in September.
The company said it had also received more than $1 billion in funding from the U.S. Biomedical Advanced Research and Development Authority, a subsidiary of the U.S. health department, to develop the coronavirus vaccine.
The British group is one of several big drug-makers racing to develop a vaccine for the virus, which has killed more than 300,000 people and crippled the global economy.
Although still at an early stage, the Oxford prototype has been one of the world’s fastest-moving potential solutions to combat the spread of the pandemic.
Some 300 million of the 400 million doses are set to go to the U.S., where the Department of Health and Human Services announced it was collaborating with AstraZeneca to make the first ones available as early as October.
The effort is part of President Trump’s Operation Warp Speed initiative, which aims to quickly scale up vaccine production in the U.S.
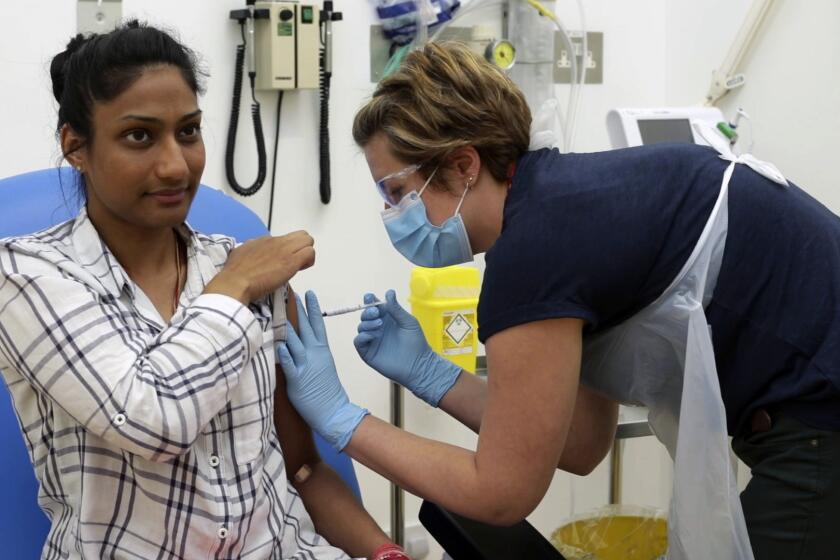
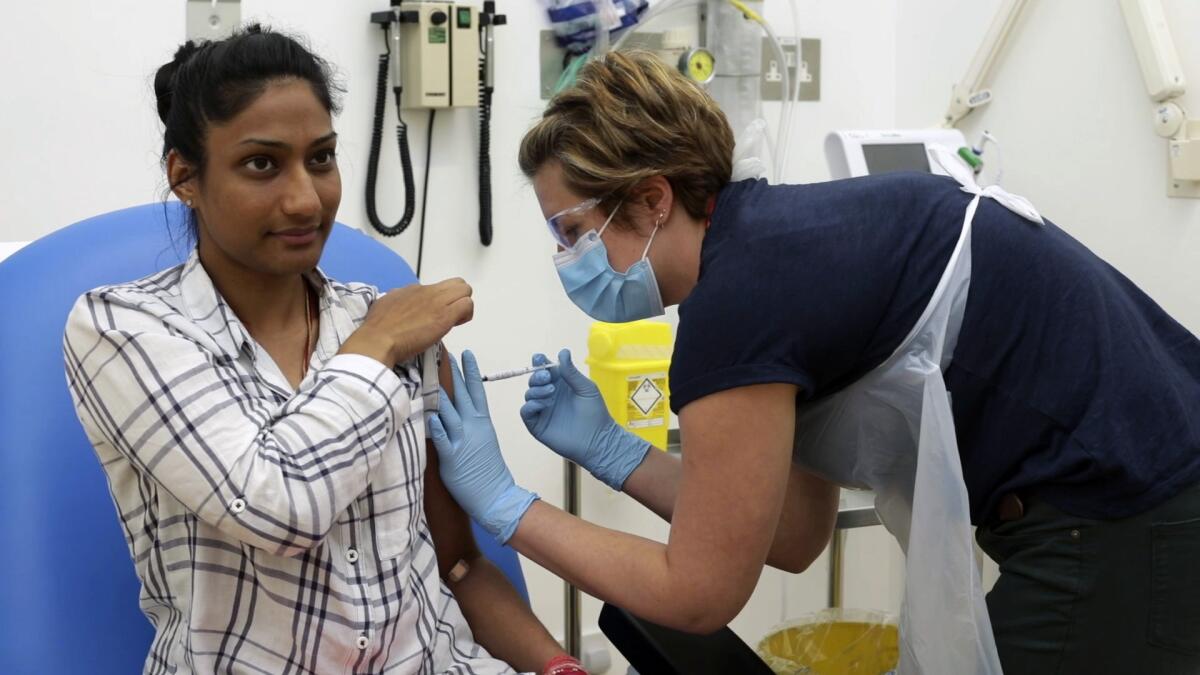
Hundreds of people are volunteering to be injected with experimental vaccines that might stop COVID-19, but there’s no guarantee any of them will work.
AstraZeneca said earlier this week that it was building a supply chain to manufacture up to 1 billion doses of the vaccine, although there is still no guarantee that the vaccine will be effective.
The Oxford vaccine began the first phase of clinical testing in healthy volunteers in late April. Earlier this week, the university said preliminary results might not be available till mid-June, when more extensive trials would begin.
The current front-runner in the global vaccine development race is U.S. company Moderna, which released some positive results earlier this week, showing that its vaccine was well-tolerated by healthy volunteers and appeared to produce the “neutralizing antibodies” needed to fight the disease. The Moderna vaccine is based on directly injecting genetic material, RNA, which then kicks the human immune system into action.
The Oxford vaccine works differently. It uses a weakened version of a common cold virus that causes infections in chimpanzees and contains the genetic material of the coronavirus that causes COVID-19. After vaccination, the recipient’s cells make proteins that prime the immune system to attack the virus if it tries to enter the body.
World leaders have pledged billions of dollars to help fund research into a coronavirus vaccine and to develop new treatments and better testing.
Its development program will involve a Phase 3 clinical trial with 30,000 participants, as well as a pediatric trial.
Drug makers have been racing to come up with a viable vaccine, compressing timelines that usually span years into months. There remains the possibility that no vaccine, including the Oxford one, may be viable.
Pascal Soriot, AstraZeneca chief executive, said: “We are so proud to be collaborating with Oxford University to turn their groundbreaking work into a medicine that can be produced on a global scale.”
Concern has been growing over so-called vaccine nationalism — the prospect of drug makers receiving support from their own governments that enables the richest nations to develop the strongest inoculation regimes.
The $1-billion U.S. investment in AstraZeneca dwarfs the $80 millon the British government said Sunday it would allocate to the Oxford project as part of an agreement to “make up to 30 million doses available by September for people in the U.K.” and up to 100 million doses altogether.
“It will mean the U.K. will be the first country to get access to the vaccine, should it be successful,” it said at the time.
But on Thursday, AstraZeneca said it was now engaging with international organizations such as the World Health Organization “for the fair allocation and distribution of the vaccine around the world.”
The WHO has backed an initiative to expand vaccine access to all countries by easing intellectual property restrictions.
AstraZeneca said it was also working with international distribution partners including the Serum Institute of India and promised that it would make the Oxford vaccine “widely accessible around the world in an equitable manner.”
Industry executives have repeatedly said that more than one effective vaccine will be necessary in order to meet the enormous demand generated by the pandemic.
© The Financial Times Ltd. 2020. All rights reserved. FT and Financial Times are trademarks of the Financial Times Ltd. Not to be redistributed, copied or modified in any way.
More to Read
Inside the business of entertainment
The Wide Shot brings you news, analysis and insights on everything from streaming wars to production — and what it all means for the future.
You may occasionally receive promotional content from the Los Angeles Times.